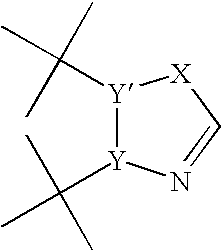
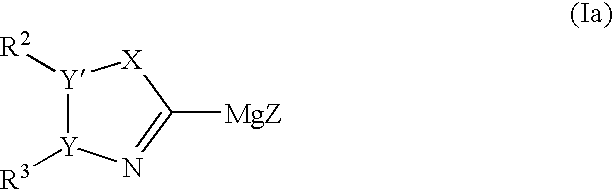Process for preparing heteroaryl and unsaturated heterocycloalkylmagnesium reagents and uses thereof
a technology of unsaturated heterocycloalkyl and reagents, which is applied in the field of new heteroaryl and unsaturated heterocycloalkylmagnesium reagents, can solve the problems of unattractive use of large-scale synthesis, and achieve the effect of less deleterious and greater yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of (S)-2-amino-1-benzoxazol-2-yl-propan-1-ol hydrochloride
[0221]
Step 1
[0222] To a solution of benzoxazole (28.6 g, 240 mmol) in toluene (150 mL) was added during ca 20 min., at ca −4° C. a 2M solution of isopropyl-magnesium chloride in THF (120 mL, 240 mmol). The red-brown mixture was stored at ca −4° C. and used as needed.
Step 2
[0223] To a solution of (S)-2-Boc-aminobutanol (50 g; 264 mmol) in dichloromethane (500 mL) and water (350 mL) were added at 20° C. TEMPO (0.01 eq), sodium bromide (1 eq) and sodium hydrogencarbonate (3 eq). The reaction mixture was stirred at 0° C. and diluted bleach (1.3 eq, 450 mL) was added over 40 min. The reaction mixture was stirred for 30 min. at 0° C. and then quenched with aq. thiosulfate. After decantation and extractions (dichloromethane), the organic phase was washed with brine, dried and concentrated in vacuo to dryness, giving (S)-2-(tert-butoxycarbonyl)-amino-butyraldehyde as a low-melting solid (38.1 g; yield: 77%). C9H17NO3;...
example 2
Synthesis of (S)-2-(tert-butoxycarbonyl)amino-1-(oxazolo[4,5-b]pyridin-2-yl)propan-1-ol
[0226]
Step 1
[0227] A mixture of 2-amino-3-hydroxypyridine (11 g, 100 mmol), triethylorthoformate (80 ml) and p-toluenesulfonic acid (61 mg) was heated at 140° C. for 8 hours. Excess triethylorthoformate was removed under vacuum and oxazolo[4,5-b]pyridine was crystalized from ethyl acetate (9 g).
Step 2
[0228] In a clean roundbottom flask equipped with stir bar was placed oxazolo[4,5-b]pyridine (600 mg, 5 mmol) in 30 mL THF and the reaction mixture was cooled to 0° C. under N2 atomosphere. Isopropylmagnesium chloride (2M in THF, 2.5 ml, 5 mmol ) was added. After stirring for 1 h at 0° C., (S)-2-(tert-butoxycarbonyl)aminobutyraldehyde (573 mg, 3 mmol) in 20 ml THF was added. The ice bath was removed and the reaction mixture was allowed to warm to room temperature. After 2 h, the reaction mixture was quenched with saturated ammonium chloride solution and concentrated to dryness. The residue was e...
example 3
Synthesis of N-[1-(R)-(1S-benzoxazol-2-ylcarbonylpropylcarbamoyl)-2-cyclopropylmethylsulfonylethyl]morpholine-4-carboxamide
[0230]
Step 1
[0231] L-Cysteine (100 g, 0.825 mol) was suspended in ethanol (850 mL). A solution of sodium hydroxide (1.65 mol) in ethanol (650 mL) was added during 40 min at 20-25° C. To the solution was added bromomethylcyclopropane (0.907 mol) at 25-30° C. The reaction mixture was stirred at ambient temperature overnight, the neutralized with 2N HCl (300 mL). The suspension was concentrated under vacuum to 400 mL, then water (750 mL) was added, and pH was adjusted to 6.5 with 2N HCl. The mixture was stirred for 2 h at 0-5° C., the precipitate was filtered, washed with water and dried under vacuum to give (R)-2-amino-3-cyclopropyl-methylsulfanylpropionic acid as a white crystalline solid (128.2 g; yield=88.6%). C7H13NO2S; MW=175.3; Tmelt=209° C.; NMR (DMSO, ppm): 0.20 (m, 2H), 0.50 (m, 2H), 0.94 (m, 1H), 2.50 (m, 2H), 2.78 (dd, J=14.5 and 8.5 Hz, 1H), 3.08 (d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com