Method for introducing functional material into organic nanotube
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
[0040] D-(+)-Glucopyranose (1.0 g, 5.55 millimoles manufactured by the Fluka Co.) was placed in a flask, and 50 ml of water was added to dissolve it. Ten grams of ammonium hydrogen carbonate (Wako Pure Chemical Industries, Ltd.) was added to the solution until crystals separated out in the bottom of the flask. The mixture was agitated using a magnetic stirrer for three to five days in an oil bath at 37° C. Ammonium hydrogen carbonate was added from time to time to maintain saturation in the reaction system. The total amount of ammonium hydrogen carbonate added was from 40 g to 50 g. The reaction was monitored using thin layer chromatography [Rf value=0.40, developing solvent: ethyl acetate / acetic acid / methanol / water (volume ratio 4 / 3 / 3 / 1)].
[0041] The reaction system was cooled to allow ammonium hydrogen carbonate crystals to separate out in a post treatment to remove unreacted ammonium hydrogen carbonate from the reaction system. A different method may be used. For example, a suita...
production example 2
[0042] A reaction system was created by placing 11-cis-Octadecenoic acid (282 mg, 1.0 millimole) (Wako Pure Chemical Industries, Ltd.) dissolved in 1 ml of dimethyl sulfoxide. HOBt (153 mg, 1.0 millimole) (Wako Pure Chemical Industries, Ltd.) and BOP (1.33 g, 3.0 millimoles) (Wako Pure Chemical Industries, Ltd.) dissolved in 1.5 ml of dimethyl sulfoxide were added to the reaction system, and the system was agitated using a magnetic stirrer for ten minutes at 25° C.
[0043] Next, the β-D-glucopyranosylamine (1.24 g, 6.9 millimoles) obtained in Production Example 1 was added to the reaction system, and the system was agitated using a magnetic stirrer for at least five hours at 25° C. to allow a reaction to occur. The reaction was monitored using thin layer chromatography [Rf value=0.56, developing solvent: chloroform / methanol (volume ratio 4 / 1)].
[0044] The crude product obtained was chromatographed using silica gel chromatography with a mixed solvent of chloroform / methanol (volume rat...
production example 3
[0047] 5 milligrams of the N-(11-cis-octadecenoyl)-β-D-glucopyranosylamine obtained in Production Example 2 was dispersed unltrasonically in 100 ml of pure water for 40 minutes. The dispersion was subsequently boiled for an hour at 110° C., allowed to cool to ambient temperature and left standing overnight.
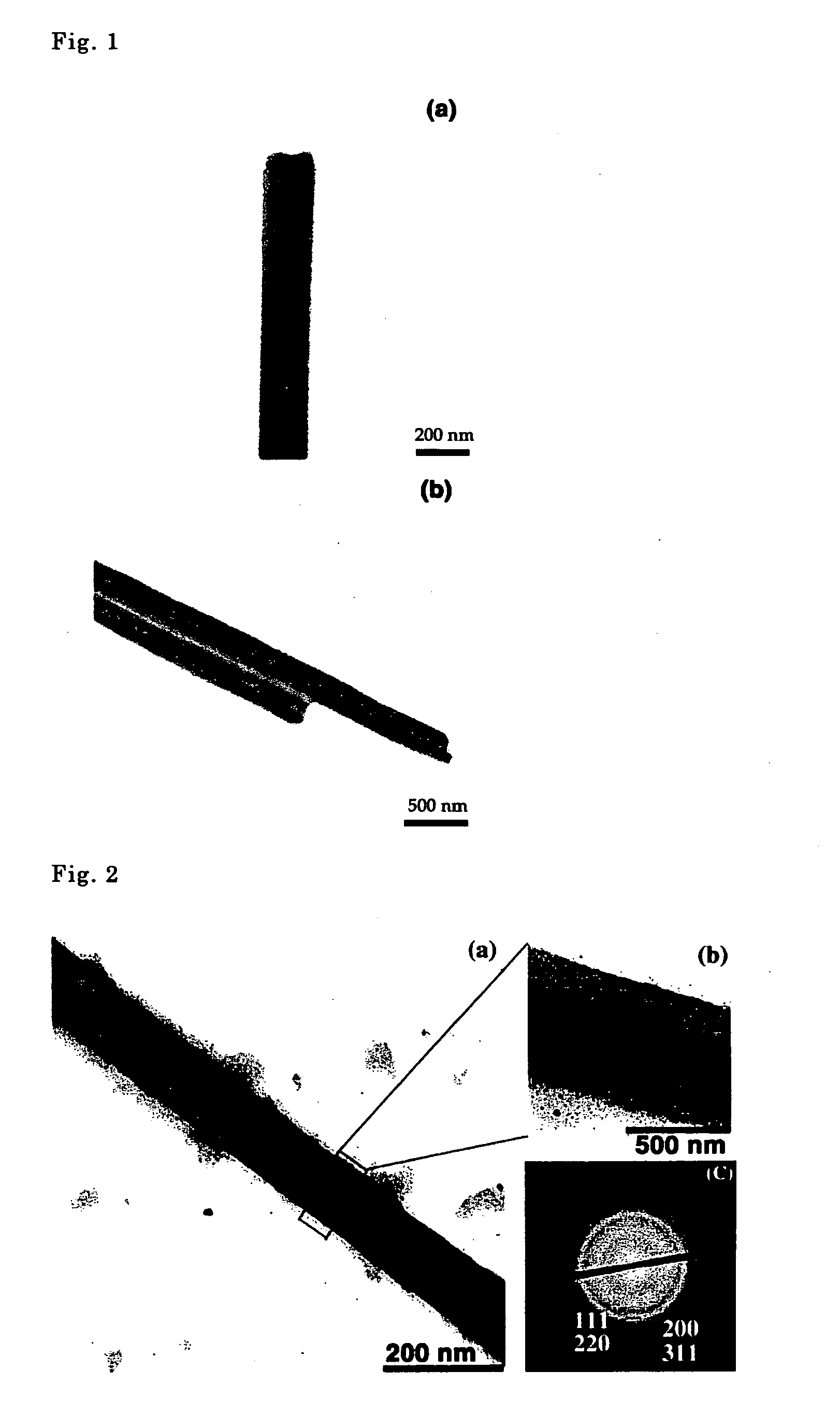
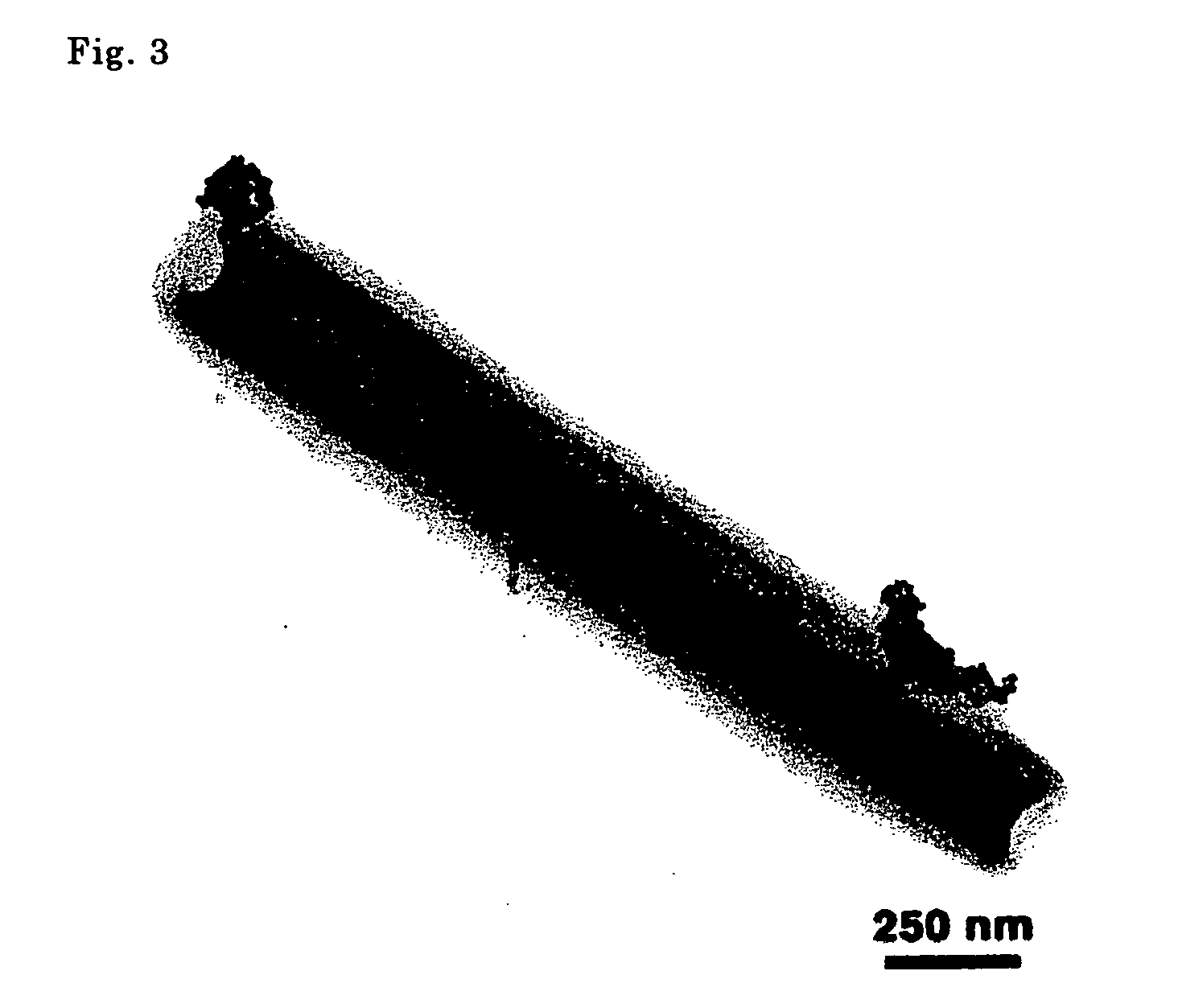
[0048] The aqueous solution obtained was examined using a transmission type electron microscope (TEM), and hollow fiber shaped organic nanotubes having an internal diameter of 45-200 nm and an external diameter of 75-500 nm were confirmed. [See FIG. 1(a).]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com