Nasal spray steroid formulation and method
a steroid and nasal spray technology, applied in the direction of biocide, drug composition, aerosol delivery, etc., can solve the problems of low bioavailability, mucosal irritation, loss of estrogen's health protective effect, etc., and achieve the effect of easing symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Solubility of 17β-Estradiol and Testosterone with 2-Hydroxypropyl-β-cyclodextrin in Water
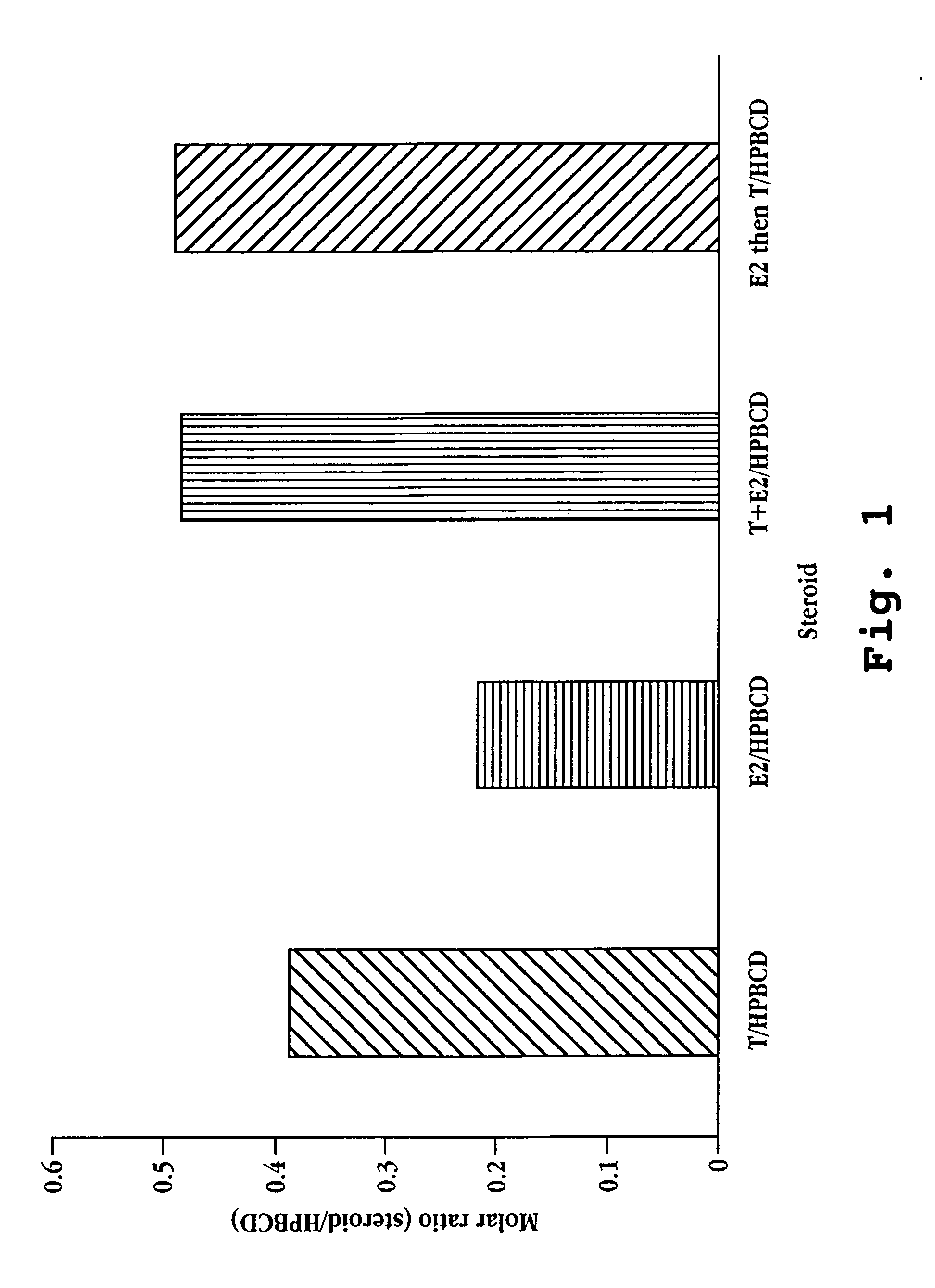
[0072] The solubility of 17β-estradiol and testosterone in varying concentrations of 2-hydroxypropyl-β-cyclodextrin (MW 1380 g / mole; 5.5 degree of substitution) was determined as follows. 10 ng 17β-estradiol (MW 272.39 g / mole) was added to 1 mL of 2-hydroxypropyl-β-cyclodextrin in water, the 2-hydroxypropyl-β-cyclodextrin concentration ranging from 10 to 250 ng / mL. In a second series of vials, 20 ng of testosterone (MW 288.43 g / mole) was added to 1 mL of 2-hydroxypropyl-β-cyclodextrin in water, the 2-hydroxypropyl-β-cyclodextrin concentration ranging from 10 to 250 ng / mL. In a third set of vials 10 ng 17β-estradiol and 20 ng testosterone were added to 1 mL of 2-hydroxypropyl-β-cyclodextrin in water, the 2-hydroxypropyl-β-cyclodextrin concentration ranging from 10 to 250 ng / mL. The vials were mixed at room temperature for about 1 hour. Aliquots were taken from the supernatant of each vial and as...
example 2
Preparation of Intranasal Formulation
[0073] 2-Hydroxypropyl-β-cyclodextrin was added to water at a concentration of 240 mg / mL and stirred until dissolved. 17β-estradiol was then added to the water-cyclodextrin solution at a concentration of 1.0 mg / mL. The mixture was stirred until dissolution. Testosterone at a concentration of 5.0 mg / mL was then added, and after stirring to dissolution benzalkonium chloride (0.1 mg / mL), ethylene diamine tetra acetic acid (EDTA; 1 mg / mL), and sorbitol (61.6 mg / mL) were added. The mixture was stirred. The volume was brought to the final desired volume and the pH was adjusted as needed. Table 6 summarizes the preparation components, concentrations, and dosages per 50 μL.
TABLE 6Components in Exemplary Nasal PreparationConcentrationComponent(mg / mL)Dose per 50 μL17β-estradiol1.050μgTestosterone5.0250μg2-hydroxypropyl-β-cyclodextrin24012mgBenzalkonium chloride0.15μgEDTA1.050μgSorbitol61.63.1mgWater, USPas required
example 3
Comparison of Intranasally and Transdermally Delivered Estradiol
[0074] Postmenopausal or surgically-postmenopausal females (n=63) were recruited for participation in the study. Thirty women were selected for treatment with transdermal 17β-estradiol from a Noven Vivelle® 50 μg / day patch. Thirty women were treated with transdermal 17β-estradiol from a Noven Vivelle-dot® 50 μg / day patch. The remaining three women were treated intranasally with a single 100 μL bolus spray containing 350 μg 17β-estradiol per spray. The spray formulation in addition to estradiol was comprised of sorbitol (61.6 mg / mL), EDTA (1.0 mg / mL), benzalkonium chloride (0.1 mg / mL), and 2-hydroxypropyl-β-cyclodextrin (100 mg / mL). Blood samples were drawn at defined intervals for analysis of serum estradiol levels. The average concentration of serum estradiol over 24 hours as pg / mL was determined and the results are shown in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| mole ratio | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com