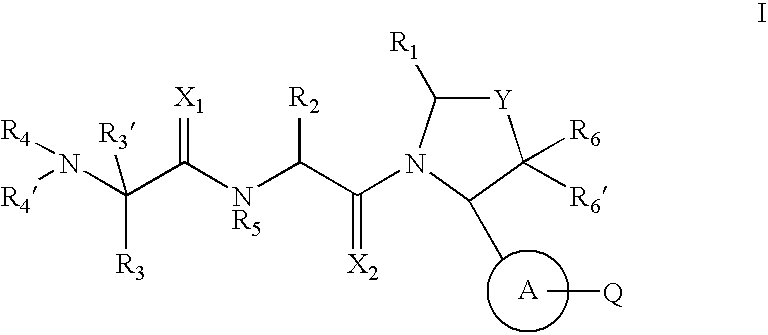
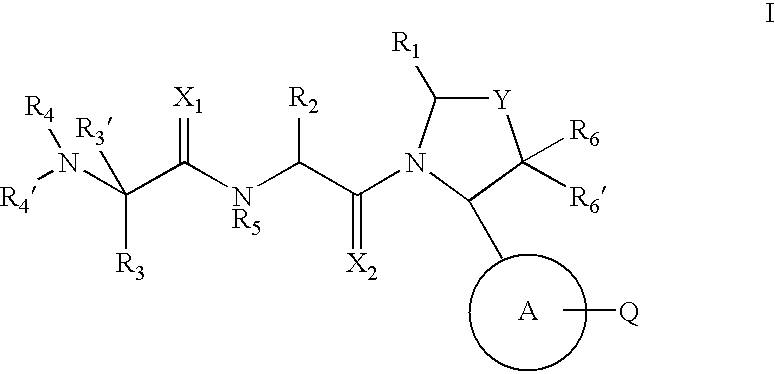
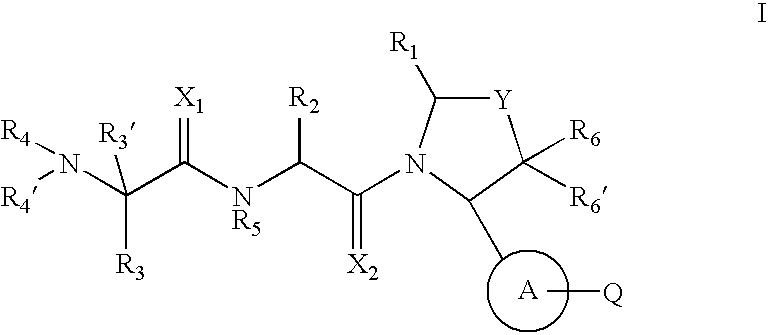
Pyrrolidine inhibitors of IAP
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2-[tert-Butoxycarbonyl-(1H-pyrrol-2-ylmethyl)-amino]-propionic acid
[0109]
[0110] Alanine ethyl ester b (5 g, 32.5 mmol), pyrrole-2-carboxaldehyde a (3.1 g, 32.5 mmol), sodium cyanoborohydride (2.04 g, 32.5 mmol) and AcOH (1%) were mixed in DMF and stirred overnight. The reaction was quenched with H2O, and DMF was evaporated. The mixture was diluted with EtOAc, washed by 0.1N NaOH, dried and concentrated to yield product c 2.5 g. The resulting ester c (2.5 g, 12.8 mmol), di-tert-butyldicarbonate (3.06 g, 14 mmol) were mixed in THF, H2O with NaHCO3 and stirred overnight. THF was evaporated, and the mixture was diluted with EtOAc, washed by 1N NaOH, sat. NH4Cl and brine. After dried, the mixture was concentrated to yield the Boc-protected ester d 3.3 g. The Boc-protected ester d (1.67 g, 5.6 mol), lithium hydroxide mono hydrate (284 mg, 6.77 mmol) were mixed in THF and H2O at 0° C. THF was vacuumed off, and the solution was acidified by dilute H2SO4, extracted by EtOAc twice. Organic l...
example 2
Thiazole Substituted Pyrrolidine
[0111]
[0112] Following the general procedure of Williams (Williams, D. R. et al, M. J. Org. Chem. 2001, 66, 8463), a mixture of N-Cbz-proline amide a (500 mg, 2.0 mmol) Lawesson's reagent (420 mg, 1.05 mmol) and toluene (5 mL) was heated at reflux for 2 h. The solution was concentrated, adsorbed onto Celite, and purified by flash chromatography (SiO2, 40% ethyl acetate-hexanes) to afford 393 mg (74%) of compound b as a colorless solid.
[0113] Following the general procedure of Ciufolini (Ciufolini, M. A. et al, J. Org. Chem. 1997, 62, 3804), ethyl bromopyruvate (200 μl, 1.43 mmol) was added to a suspension of thioamide b (378 mg, 1.43 mmol) in ethanol (5 mL), and the mixture heated at 80° C. for 5 min. The solvent was evaporated under reduced pressure, and the residue purified by flash chromatography (SiO2, gradient elution, 30-40-50% ethyl acetate-hexanes) to afford 393 mg (74%) of thiazole c as a colorless solid.
example 3
Oxazole Substituted Pyrrolidine
[0117]
[0118] A mixture of N-Boc-proline a (5.35 g, 24.9 mmol) serine methyl ester hydrochloride b (3.50 g, 22.5 mmol), EDC (4.76 g, 24.85 mmol), DIPEA (4.0 mL, 22.5 mmol) and CH2Cl2 (90 mL) was maintained overnight. The mixture was diluted with CH2Cl2 (200 mL) and washed with 1 N HCl (3×100 mL), 0.1 N NaOH (3×100 mL) and brine (1×100 mL). The organic layer was dried (Na2SO4), filtered, and concentrated to afford 5.2 g (73%) of dipeptide c as a colorless foam.
[0119] To a cool (0° C.) solution of dipeptide c (4.57 g, 14.4 mmol) and THF (100 mL) was added Burgess Reagent (Pihko, P. M.; Koskinen, A. M. P.; Nissinen, M. J.; Rissanen, K. J. Org. Chem. 1999, 64, 652, and references therein) (3.77 g, 15.8 mmol) in 3 portions over 30 min. The cooling bath was removed and the reaction allowed to reach rt, then heated at reflux for 1 h. After cooling to rt, the THF was removed under reduced pressure and the residue was partitioned between EtOAc (200 mL) and sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com