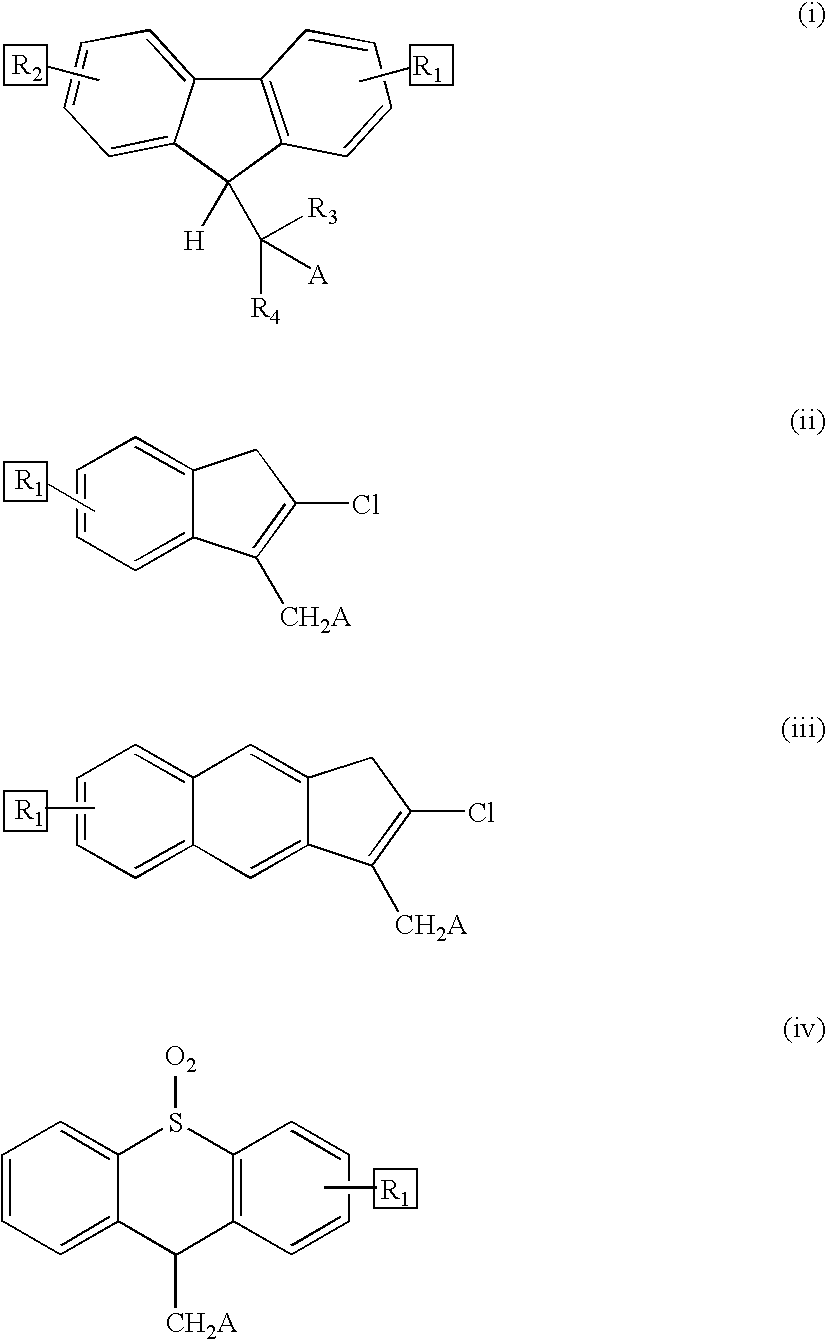
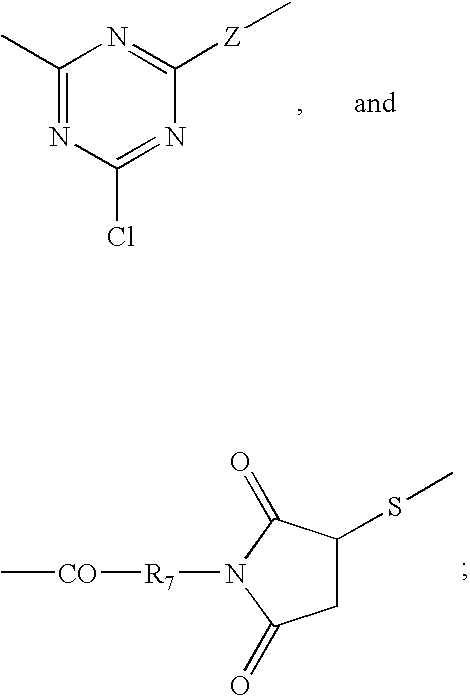
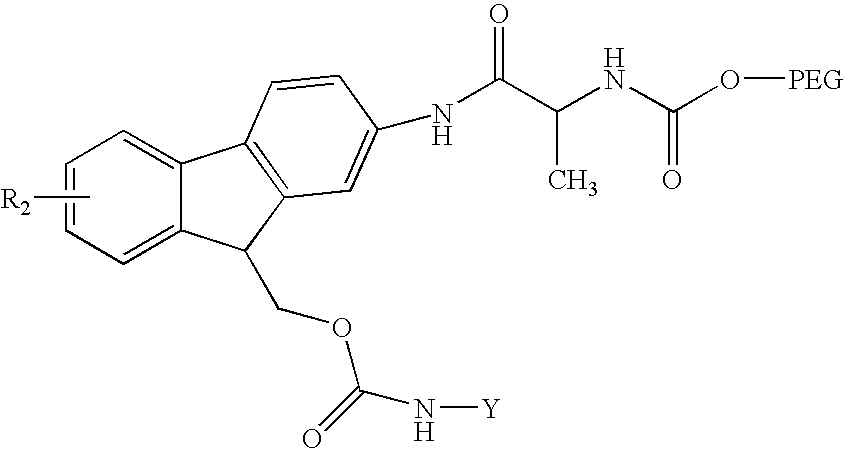
Reversible pegylated drugs
a pegylated drug and reverse pegylation technology, applied in the field of reversible pegylation of drugs and to pegylated drugs, can solve the problems of major potential drawbacks, drastic loss or even abolishing of biological and pharmacological potencies of proteins, and the technology suffers from a principal drawback, and achieves the effect of prolonging the half-life of the circulating cycl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of PEG5,000-Fmoc-OSu (Precursor 1)
[0216] Precursor 1 of the formula depicted in Scheme 1 was prepared starting from 2-aminofluorene and t-Boc-alanine (BocAla) by several steps, as depicted in Scheme 2.
1(a). Synthesis of 2-(t-BocAla-amino)fluorene (Intermediate a)
[0217] t-Boc-Ala (4.16 gr, 22 mmol) was dissolved in 11 ml dioxane. N,N′-Dicyclohexylcarbodiimide (DCC) (11 mmol in 11 ml 1 M DMF) was then added and the reaction was carried out for 3 hours at 25° C. under stirring. Dicyclohexylurea (DCU) formed was removed by centrifugation. The symmetrical anhydride product thus obtained (t-Boc-Ala-anhydride) was reacted overnight under stirring with 2-aminofluorene (0.925 g, 11 mmol) in 30 ml dioxane-water (1:1, v:v) containing 11 mmol of NaHCO3. The white solid formed was collected and dried under P2O5 in vacuum for 24 hours. Intermediate a was obtained in 60% yield (1.31 g, 3.34 mmol). It migrated on TLC (dichloromethane) with Rt=0.17. Mass-spectroscopy revealed a mass of ...
example 2
Synthesis of PEG5,000-FMS-OSu (Precursor 2)
[0224] Precursor 2, PEG5,000-FMS-OSu, depicted in Scheme 1, was prepared by sulfonation of Precursor 1, PEG5,000-Fmoc-OSu, with chlorosulfonic acid, as depicted in Scheme 2. Briefly, to a solution of Precursor 1 in 4.0 ml CH2Cl2, cooled to 0° C., a solution of ClSO3H in CH2Cl2 was added dropwise over a period of 15 min and the solution was stirred for 2 hours at 25° C. The product, PEG5,000-FMS-OSu, sulfonated at position 2 of the fluorene ring, was purified by preparative HPLC-procedure, and characterized by mass spectroscopy, elementary analysis (for sulfur), following extensive dialysis, and for its rate of hydrolysis (at pH 8.5, 37° C.) following conjugation to either gentamicin or insulin.
example 3
Synthesis of PEG40000-Fmoc-OSu (Precursor 3)—
[0225] Precursor 3, PEG40000-Fmoc-OSu, depicted in Scheme 1, was prepared as described in Example 1, steps 1(e) and 1(f), but replacing PEG5000-OSu with PEG40000-OSu under the same reaction conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com