Amine-containing compound analysis methods
a compound analysis and compound technology, applied in the field of amine-containing compound analysis methods, can solve the problems of hplc having drawbacks, difficult and time-consuming derivatization step performing before analysis, and the absolute quantitation of amine-containing compounds by the above-mentioned methods can be problemati
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Compounds from Different Sample Types
[0111] The following example illustrates the use of a variety of sample types. The teachings of this example are not exhaustive, and are not intended to limit the scope of these experiments or the present teachings
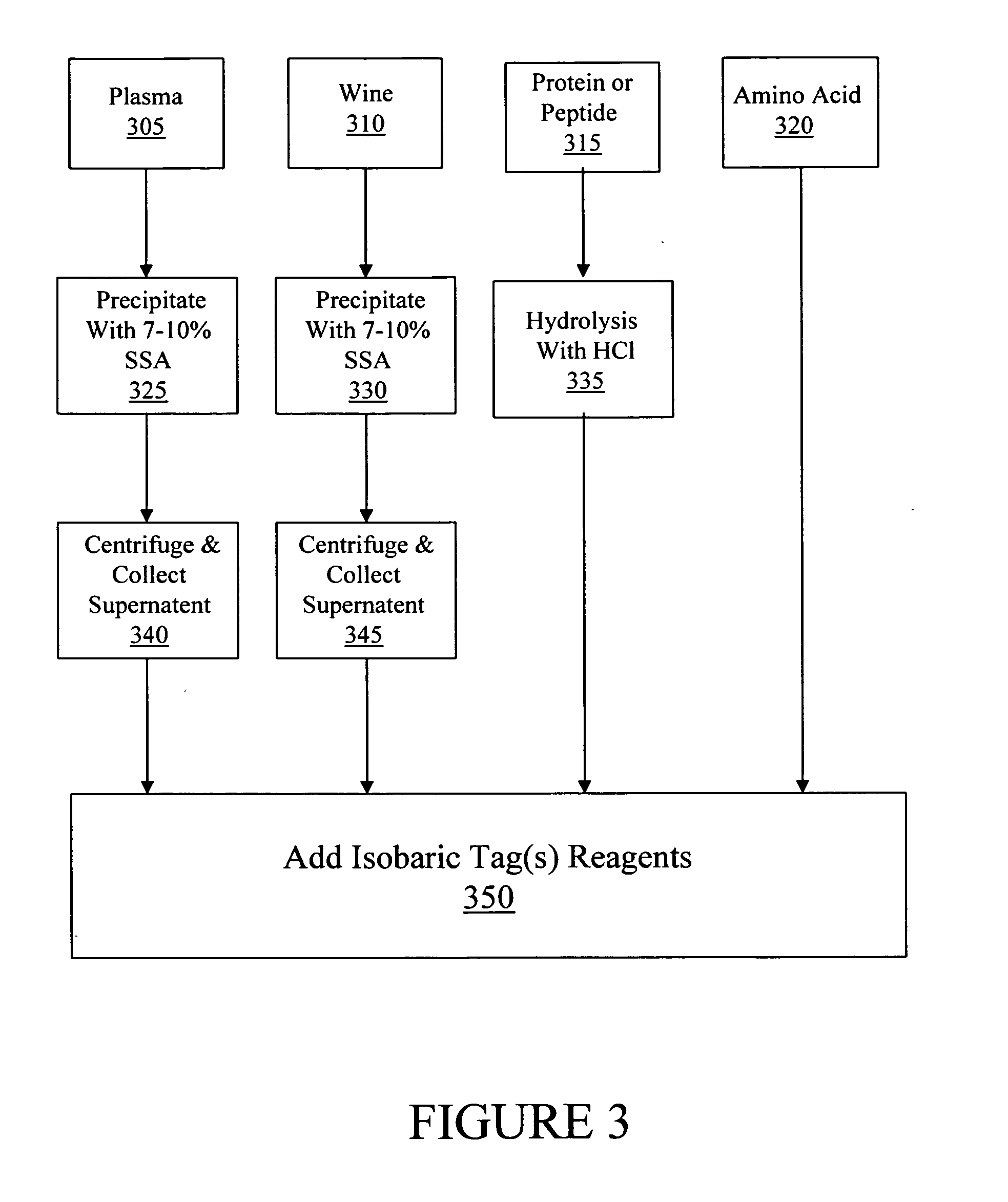
[0112] Various embodiments of the analysis of one or more amine-containing compounds from various sample types using a method of the present teachings are illustrated. In various embodiments, the one or more of amine-containing compounds of interest can originate from one or more diverse sample types, e.g, plasma, wines, proteins, peptides and amino acids in this example. In general, a sample can be processed such that compounds of interest in a sample contain one or more amine groups suitable for labeling with an isobaric tag, such as, for example, primary and secondary amines.
[0113] Referring to FIG. 3, in various embodiments, the labeling of one or more amine-containing compounds contained in a sample of plasma (block 305) compris...
example 2
Sample Preparation and Labeling with iTRAQ™ Brand Reagents
[0117] The following example illustrates examples of various embodiments preparaing and labeling one or more samples comprising one or more proteins or peptides with one or more isobaric tags using iTRAQ™ brand reagents. The teachings of this example are not exhaustive, and are not intended to limit the scope of these experiments or the present teachings.
[0118] Reduction and of Protein or Peptide Samples and Cysteine Blocking
[0119] Referring to FIG. 4A, in various embodiments, to each of at least one and up to four sample tubes, each containing between 5 and 100 μg of protein, is added 20 μL Dissolution Buffer and 1 μL Denaturant, followed by vortexing to mix (step 405). To each sample tube, 2 μL Reducing Reagent is added, followed by vortexing to mix and incubation at 60° C. for 1 hour (step 410). Spinning each sample tube is followed by addition of 1 μL Cysteine Blocking Reagent to each sample tube, then mixing by vortex...
example 3
Combining with a Standard Compound
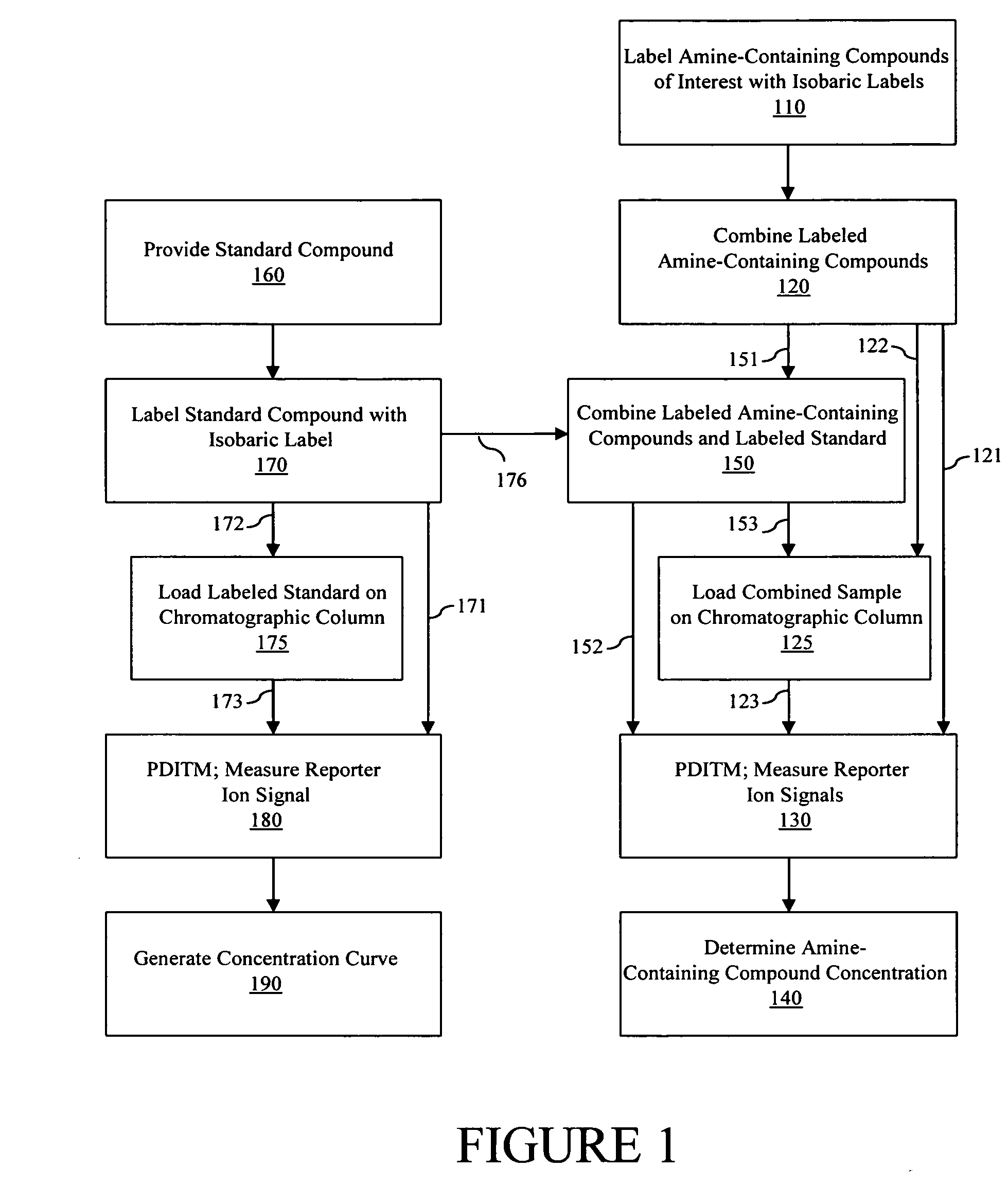
[0130] Referring to FIG. 5, in various embodiments, the determination of the concentration of one or more amine-containing compounds in one or more samples can proceed with providing one or more amine-containing compounds, including, but not limited to peptides, polypeptides, proteins, amino acids, nitrofuran metabolites, polyamines and catecholamines. In various embodiments, an isobarically labeled standard compound (step 505) (e.g., an amine-containing compound of interest from a control sample, an amine-containing compound of interest from a sample of known concentration, etc.) is used as an internal standard (e.g., combined with) two or more isobarically labeled amine-containing test compounds to be analyzed, e.g., test compound #1 (step 510), test compound #2 (step 520), and test compound #3 (step 525).
[0131] In various embodiments, two or more of the amine containing compounds to be analyzed comprise the same amine-containing compound of int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| MW | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com