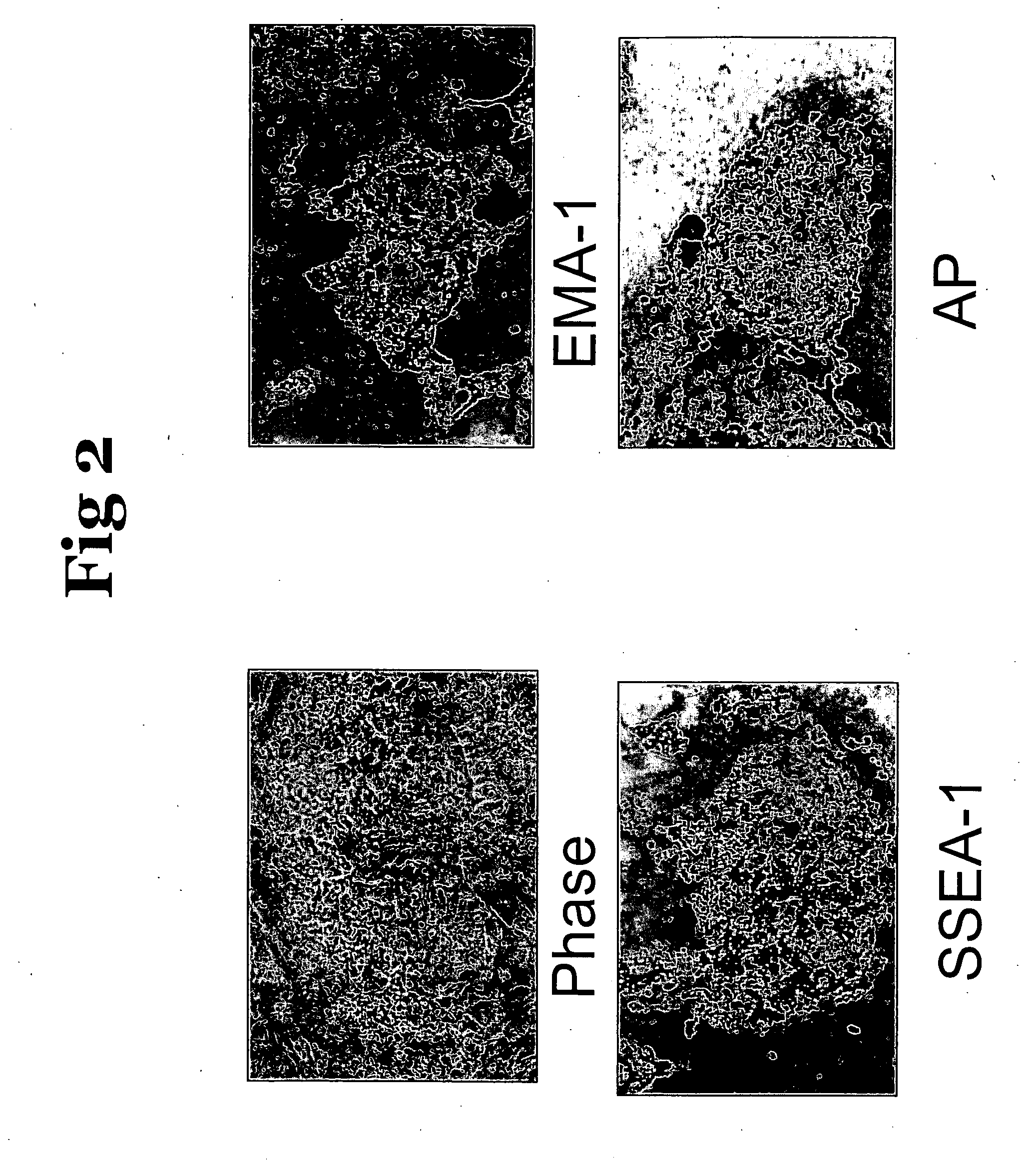
Tissue specific expression of antibodies in chickens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Derivation of Chicken Embryonic Stem Cells (cES Cells)
[0033] Chicken ES cells were derived from one of two crosses: Barred Rock X Barred Rock or Barred Rock X Rhode Island Red. These breeds were selected to obtain a feather marker when testing the developmental potential of cES cells. The cES cells are injected into White Leghorn embryos, which are homozygous dominant at the dominant, white locus. Chimeric chickens resulting from injection of these ES cells display black feathers from the cES cells and white feathers from the recipient embryo.
[0034] Initial establishment of the cES cell culture was initiated according to the protocol described in U.S. Pat. No. 5,565,479. At stage X, the embryo is a small round disk, consisting of approximately 40,000-60,000 cells, situated on the surface of the yolk. To retrieve the embryo a paper ring is put on the yolk membrane, exposing the embryo in the middle. The yolk membrane is cut around the ring, which is then lifted off the yolk. The em...
example 2
Injection of Chicken Embryonic Stem Cells into Recipient Embryos
[0048] To permit access to the embryo in a freshly laid egg the shell must be breeched, inevitably leading to a reduction in the hatch rate at the end of the 21-day incubation period. The convention was to cut a small hole (less than 10 mm diameter) in the side of the egg, through which the embryo was manipulated, and re-seal with tape, a glass cover slip, shell membrane or a piece of shell. Though relatively simple to perform, this “windowing” method caused embryonic mortality between 70 and 100%. Improved access to the embryo and increased survival and hatchability can be achieved if the embryo is transferred to surrogate eggshells for incubation to hatching using two different shells and a method (adapted from Callebaut) (Callebaut, Poult. Sci 60: 723-725, 1981) and (Rowlett, K. and K. Simkiss, J. Exp. Biol. 143: 529-536, 1989), which are specifically incorporated herein by reference with this technique, the mean ha...
example 3
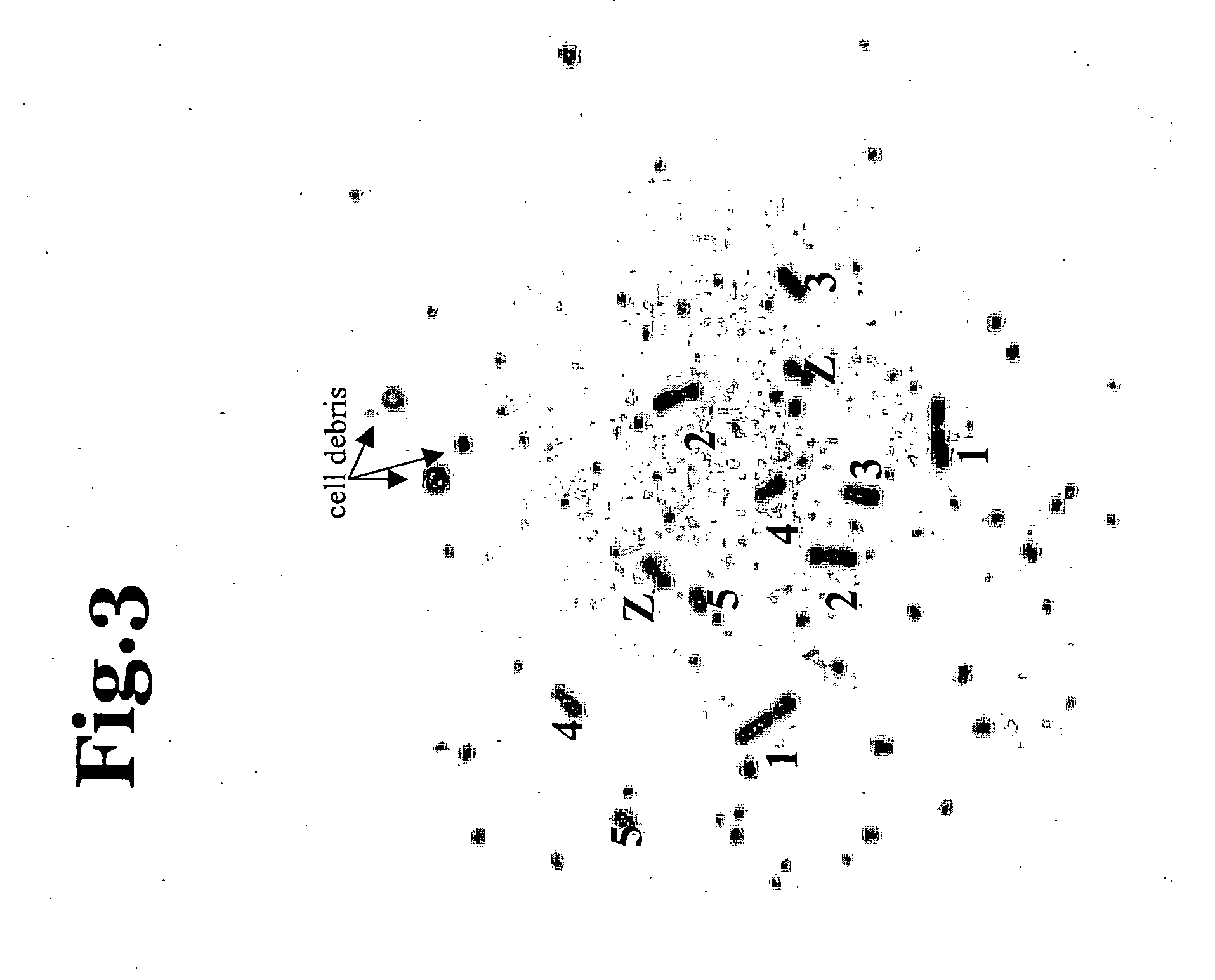
Somatic Chimeras from Chicken Embryonic Stem Cells (cES)
[0057] Chicken ES cells are injected into White Leghorn recipient embryos. In the first round of experiments, a total of 14 cell lines in 28 experiments are injected into stage X recipient embryos (See Table 2). The cES cells have been propagated in culture between 4 and 106 days and some lines had been cryopreserved. Chicken ES cells are lightly trypsinized, resulting in small clumps of cES cells, and resuspended in DMEM supplemented with 25 mM HEPES+10% fetal calf serum. Three to five μl of the cell suspension, containing between 2000-5000 cells, are injected into the subgerminal cavity of the recipient embryos. All embryos that developed feathers are analyzed and twenty four percent of embryos (83 / 347) are chimeric as determined by feather color. Feather chimeras are obtained from 11 / 14 cell lines. The extent of chimerism varied from 1%-95% with a mean extent of 25.9% (SD=20.4).
[0058] Table 2 illustrates the variance in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com