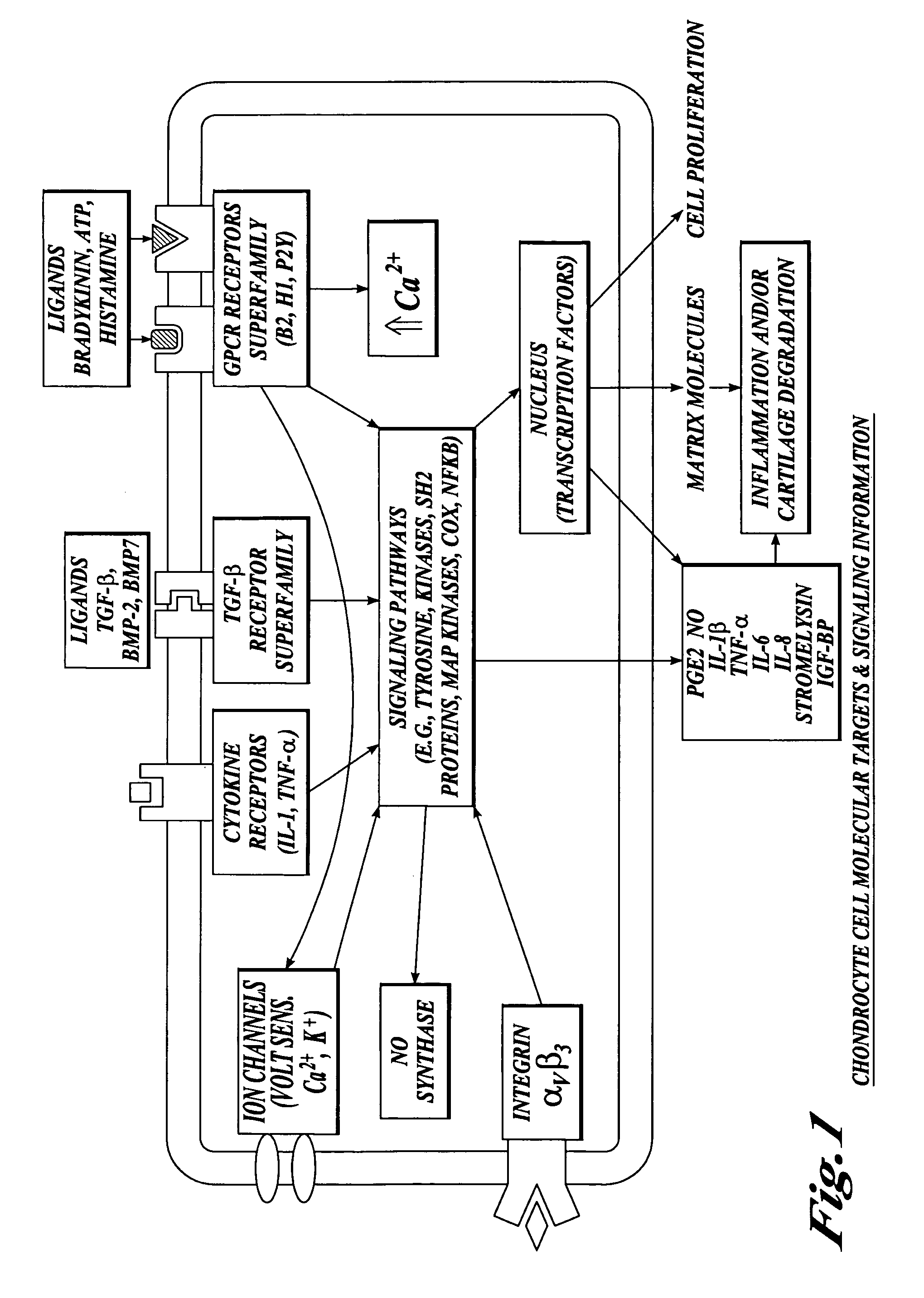
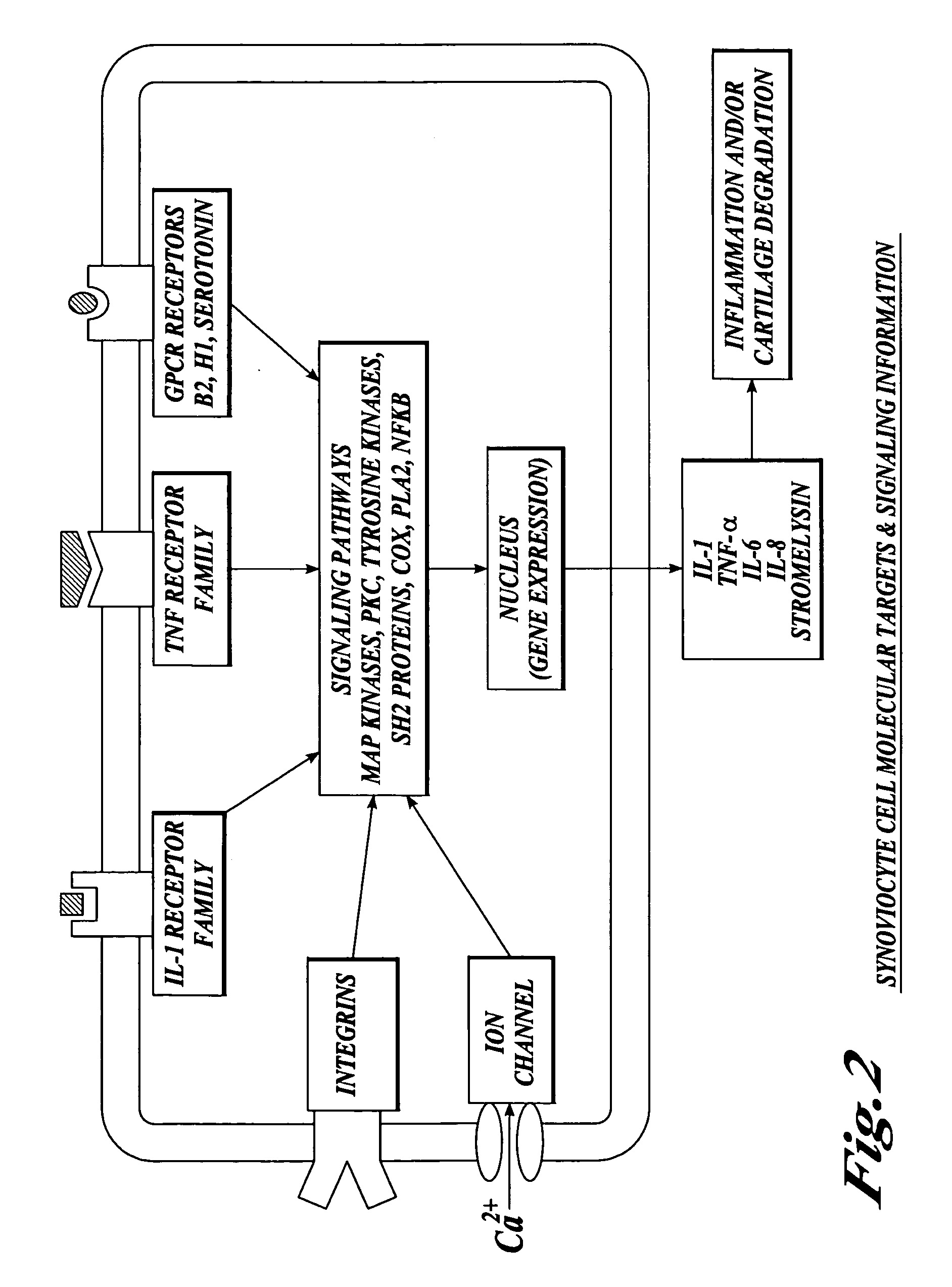
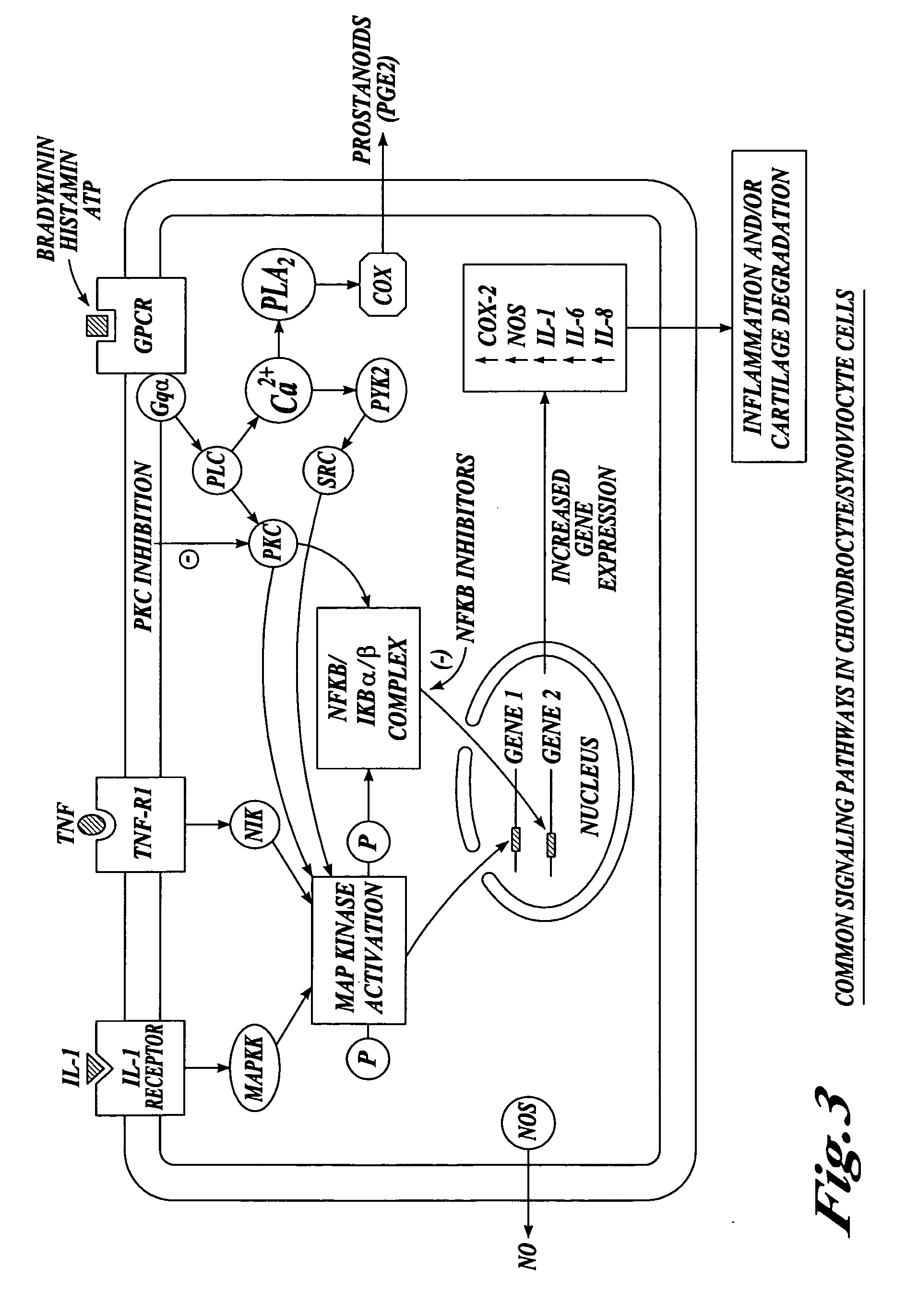
Compositions and methods for systemic inhibition of cartilage degradation
a technology of compositions and methods, applied in the direction of drug compositions, powder delivery, peptides, etc., can solve the problems of cartilage destruction process, irreversible impairment of joint motion, and no therapeutic regimen has been proven to retard the progression of articular cartilage degradation, etc., to improve dosage control, reduce the usefulness of these agents, and reduce the cost of these active agents per procedure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Irrigation Solution for Arthroscopy
[0346] The following composition is suitable for use in anatomic joint irrigation during arthroscopic procedures. Each drug is solubilized in a carrier fluid containing physiologic electrolytes, such as normal saline or lactated Ringer's solution, as are the remaining solutions described in subsequent examples.
ConcentrationClass of AgentDrug(Nanomolar)MAP Kinase InhibitorSB203580200Matrix MetalloproteinaseU-24522200InhibitorTGF-β AgonistTGF-β2200
example 2
Alternative Irrigation Solution for Arthroscopy
[0347] The following composition is an alternate formulation suitable for use in anatomic joint irrigation during arthroscopic procedures.
ConcentrationClass of AgentDrug(Nanomolar)MAP Kinase InhibitorSB203580200Nitric Oxide SynthaseL-NIL1,000InhibitorInterleukin ReceptorIL-10100Agonist
example 3
Alternate Irrigation Solution
[0348] The following drugs and concentration ranges in solution in a physiologic carrier fluid are suitable for use in the present invention.
ConcentrationClass of AgentDrug(Nanomolar)MAP Kinase InhibitorSB242235200Nitric Oxide SynthaseL-NIL10,000InhibitorTGF-β AgonistTGF-β2100
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com