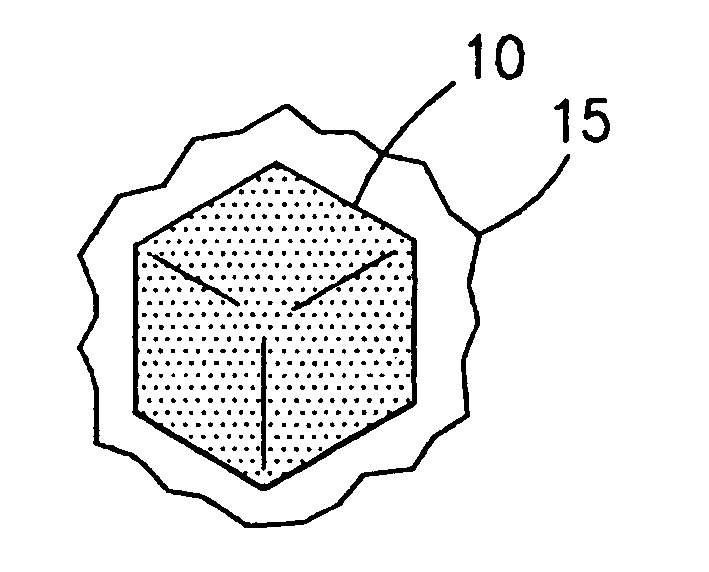
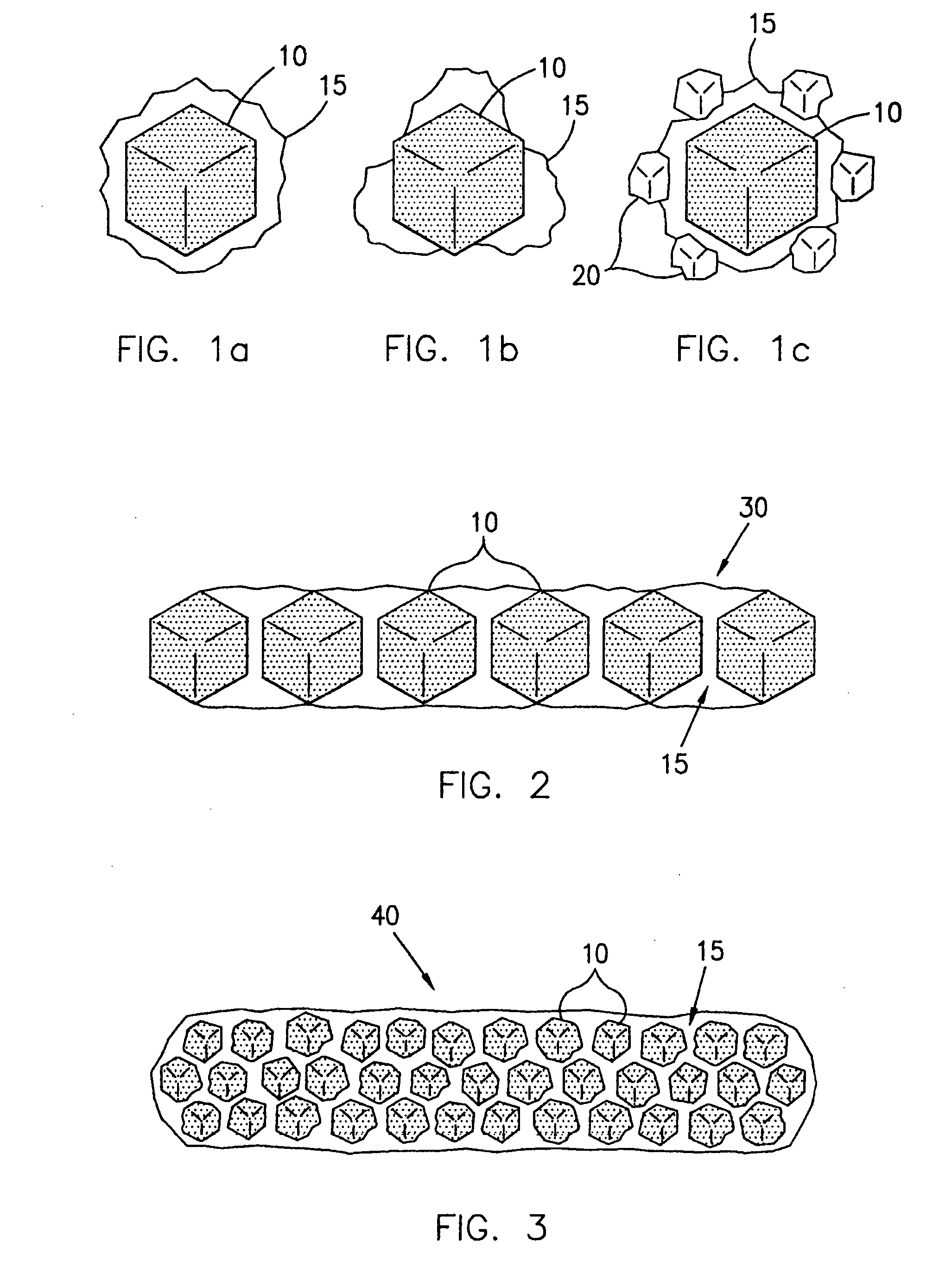
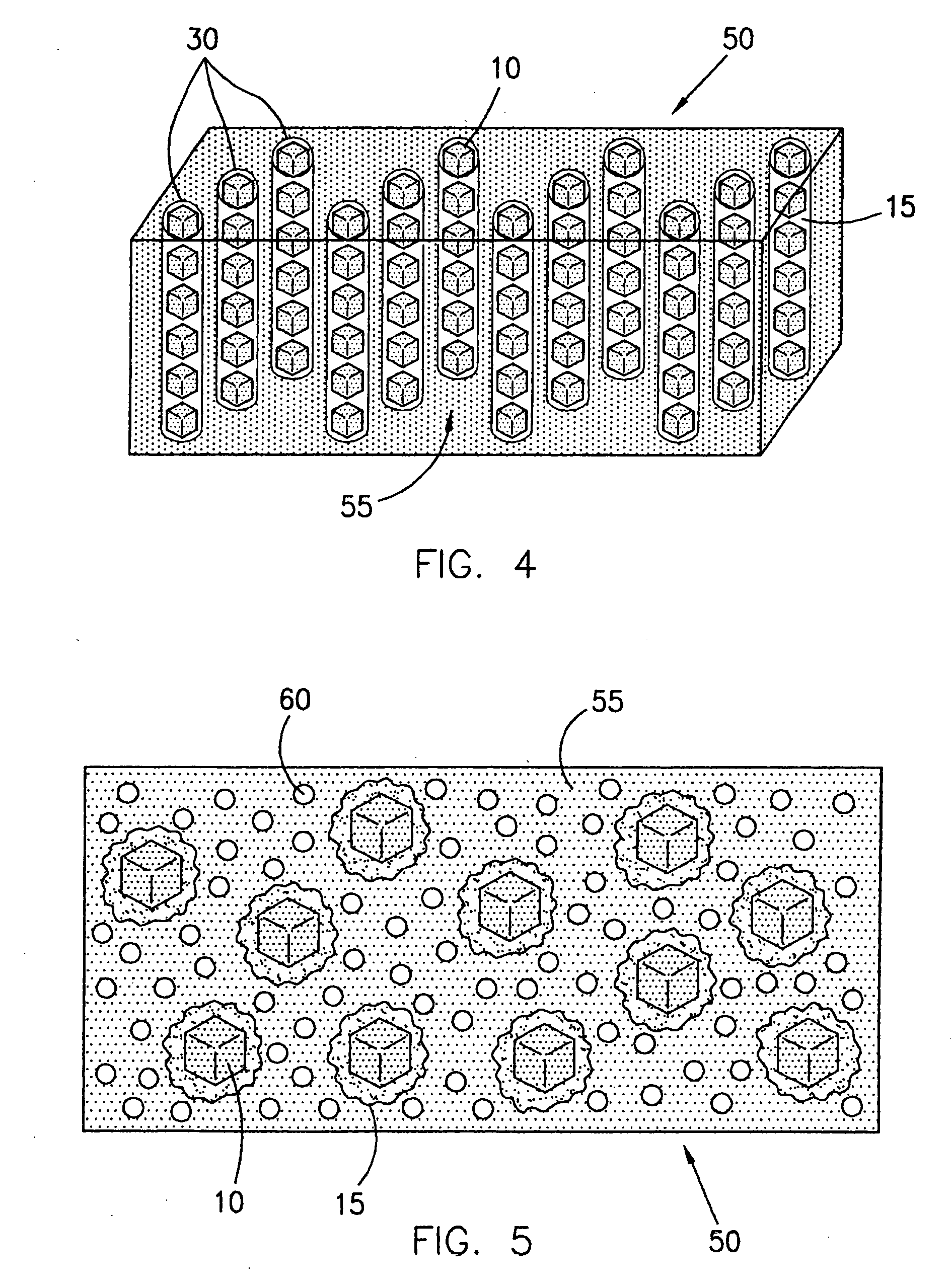
Methods of maximizing retention of superabrasive particles in a metal matrix
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0110] Diamond particles of 40 / 50 mesh were covered with a thin film of an acrylic binder.
[0111] The binder covered diamond was then mixed with a powdered metallic alloy containing B, Ni, Cr, Si, having an average particle size of about 325 mesh, and sold under the trade name NICHROBRAZ LM® (Wall Colomnoy). The result was a braze powder wrapped diamond. These coated particles were then mixed with fine powder of Al2O3. The mixture was heated in a vacuum furnace held at 10−5 torr to a maximum temperature of about 1005° C. for approximately 17 minutes to assure that the metallic alloy coating became molten and liquefied and flowed around the diamond particles wetting them. The mixture was then cooled and retrieved from the furnace. After separating the diamond particles from Al2O3, a number of coated particles were mixed with a cobalt powder and sintered in a hot press to form rectangular segments. Some of these segments were broken by bending with pliers. The fractured surface was th...
example 2
[0112] The same procedure as outlined in Example 1 was followed, but the Al2O3 separator powder was replaced with diamond particles having an average mesh size of from about 325 to about 400 mesh. During the heating process, the smaller diamond particles were wetted by the braze alloy coating, and became chemically bonded to the outside of the coated diamond particle. Thus, coated diamond particles having a chemically bonded metallic alloy shell with smaller diamond particles further bonded to the outside of the shell were produced. These “spiky” coated particles were incorporated into a cobalt matrix and fracture tested as above with similar results achieved.
example 3
[0113] The process of Example 2 was followed, but the smaller diamond particles were replaced with particles of SiC. The process yielded a coated diamond particle having ceramic particles bonded to the outside of the metallic coating similar to the diamond particles of Example 2. Moreover, the fracture testing yielded results similar to that of Examples 1 and 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com