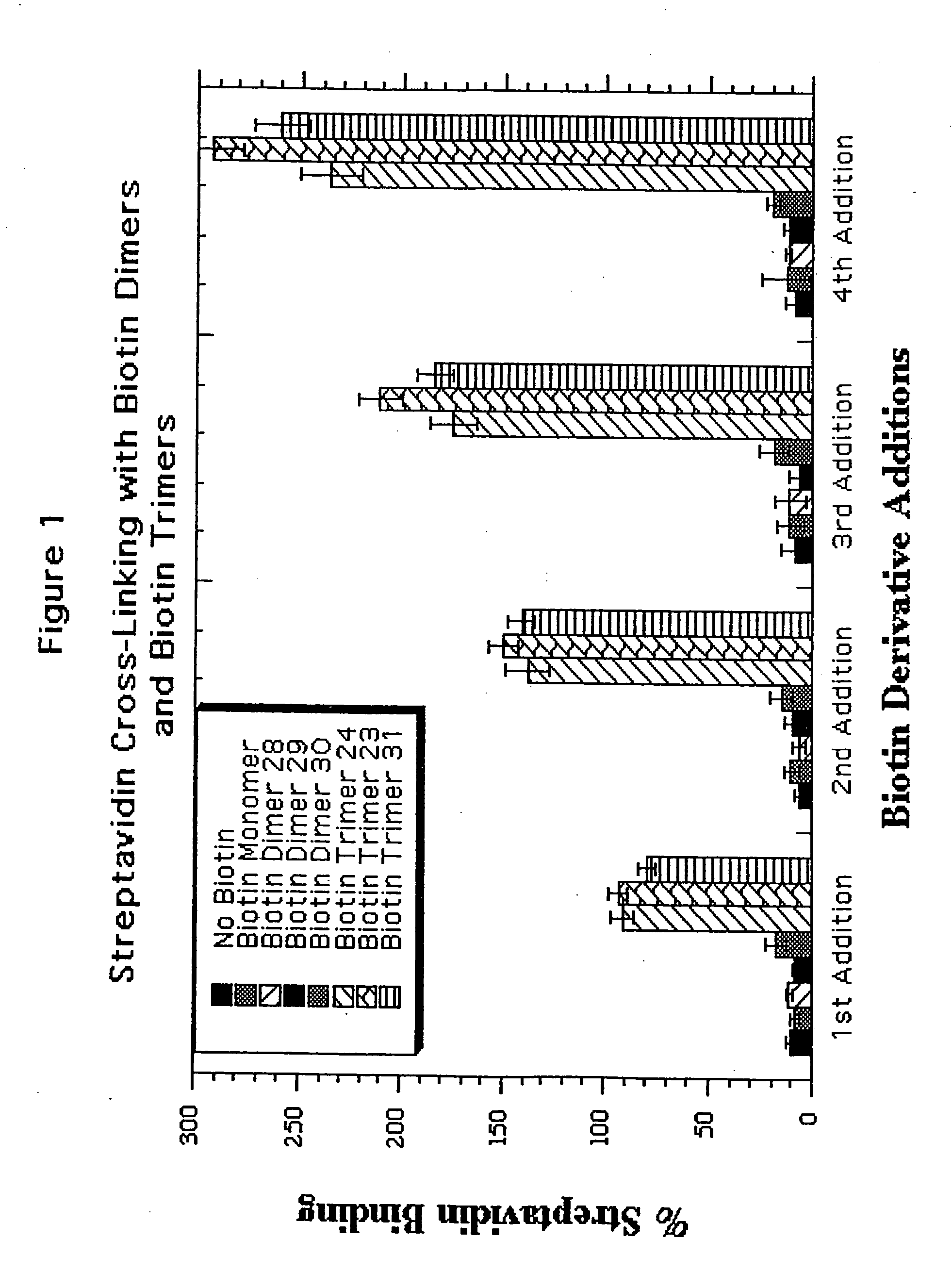
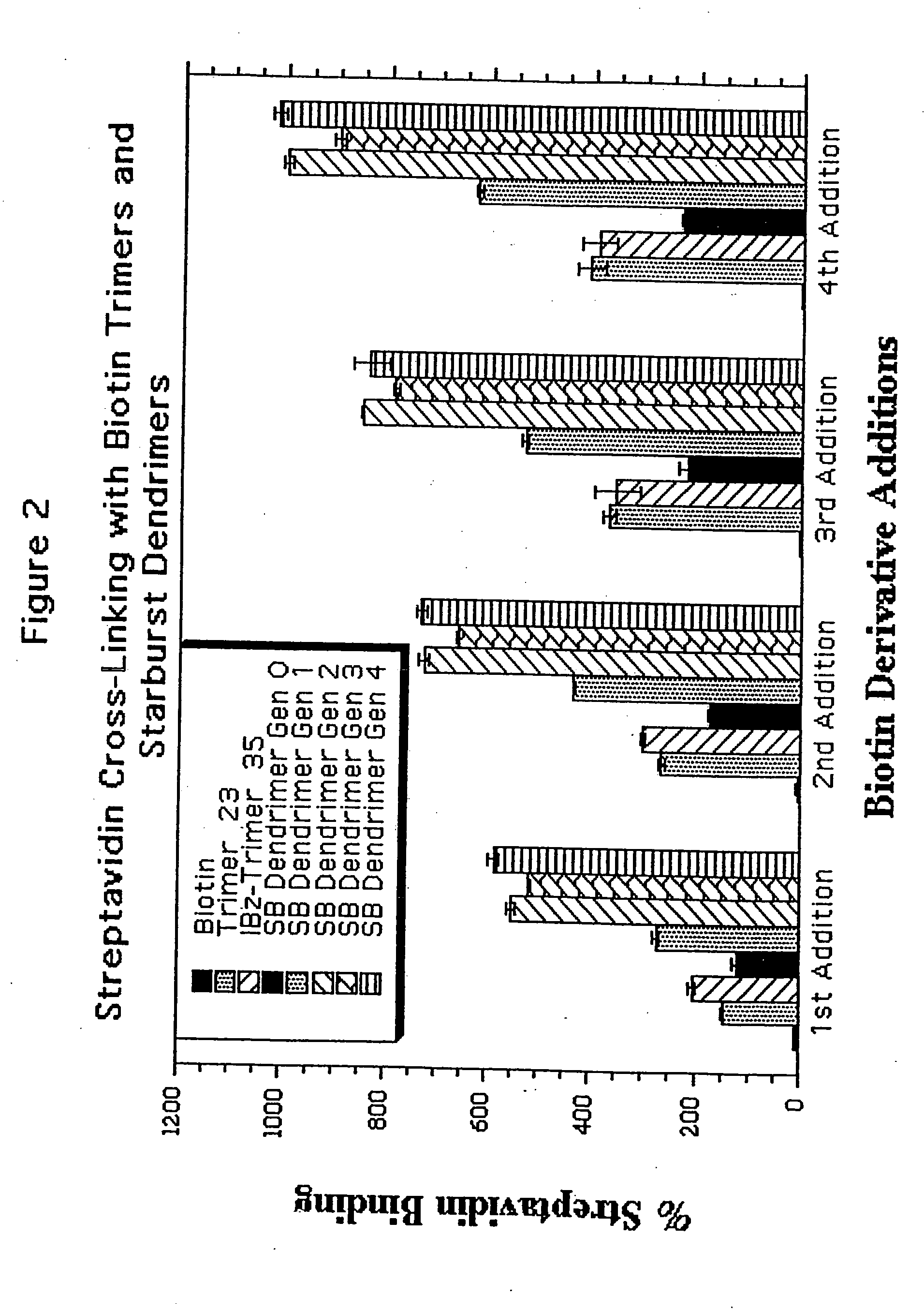
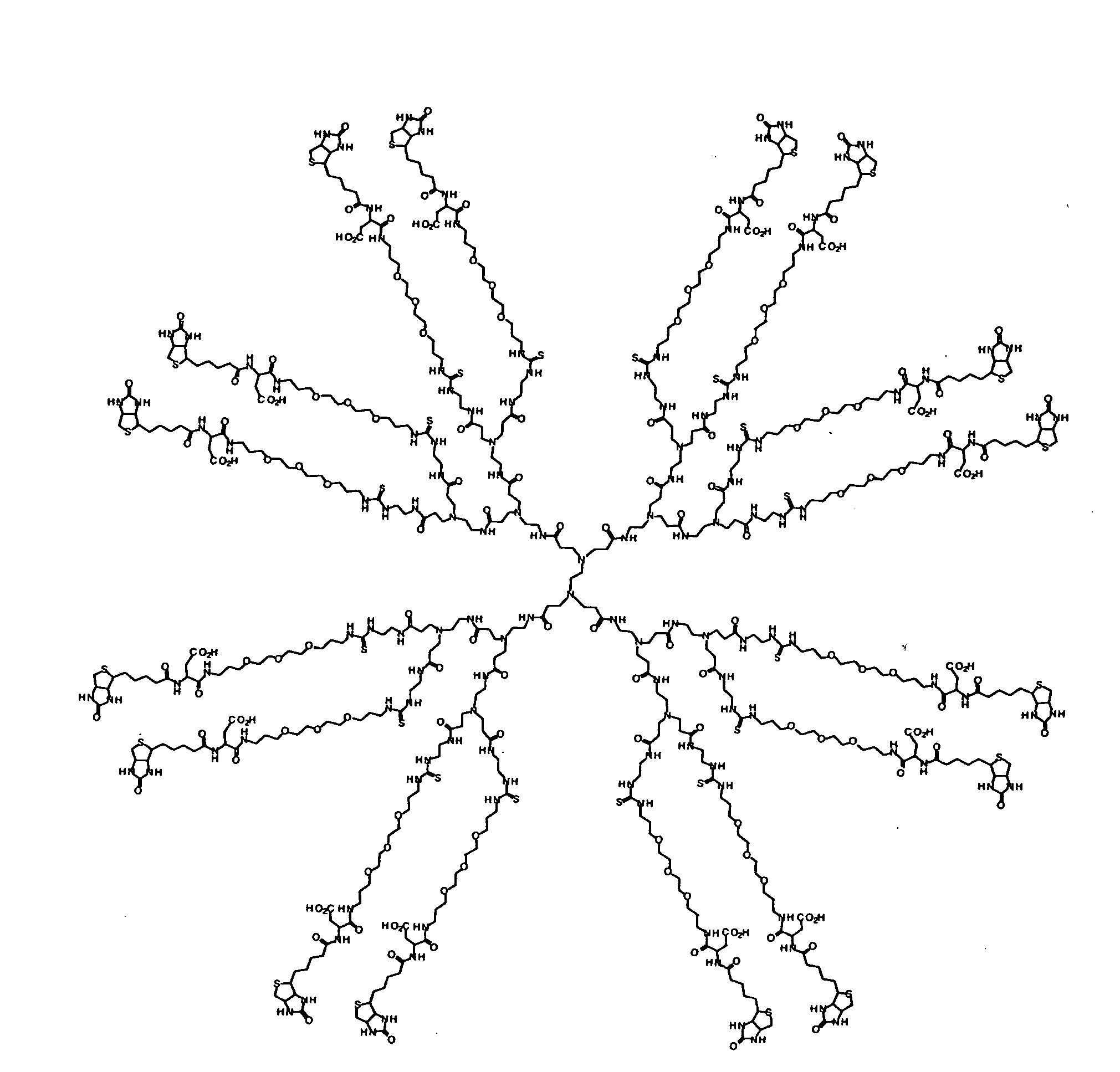
Water soluble multi-biotin-containing compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method for Synthesizing an Activated Ester of a Biotin Moiety
[0090] The following Example sets forth a methodology for preparing a tetrafluorophenyl (TFP) ester of a biotin moiety that is subsequently reacted with a water-soluble linker moiety and then a cross-linker of at least tri-functionality to produce a biotin-containing compound according to the invention. Other methods for preparing activated esters generally known in the field can be used to prepare biotin activated esters containing any number of phenolic and other hydroxyl (e.g., N-hydroxylsuccinimide) groups.
Preparation of Biotin Tetrafluorophenyl Ester
[0091] Biotin (10 g, 40.9 mmol) was dissolved in 200 mL warm (70° C.) DMF under an argon atmosphere. The solution was allowed to cool to ambient temperature and 10 mL (82 mmol) triethylamine was added, followed by the addition of 16 g (61 mmol) of 2,3,5,6-tetrafluorophenyl trifluoroacetate. The reaction was stirred at room temperature for 30 min and solvent was remove...
example 2
Method for Preparation of a Water-soluble Biotinylation Reagent
[0092] This Example sets forth a general methodology for preparing a biotin moiety linked to a water-soluble linker moiety. In the example, a diamino-ether linker is used. Ether linkers containing terminal functionalities such as, an amine and a carboxylate; an amine and an alcohol; an alcohol and a carboxylate; and two alcohols, can also be prepared using the method described. The general method can also be used when the linker contains polyhydroxyl groups if the terminal functionalities are two amines, or are an amine and a carboxylate.
Preparation of A Biotin Compound Containing A Trioxo-Amide Linker With An Amine Terminus
[0093] The TFP ester of biotin (5 g, 12.8 mmol) (Example 1) was dissolved in 200 mL anhydrous DMF. In another flask containing 28 g (128 mmol) 4,7,10-trioxa-1,13-tridecanediamine was added 4 mL of triethylamine. Both the flasks were cooled to 0-5° C. by ice water. The TFP ester of biotin was adde...
example 3
Reacting an Activated Ester of Biotin with a Diamino-linker that has One Amine Protected
[0094] This Example sets forth a general methodology for preparing a biotin / water-soluble linker adduct wherein one amine group was protected, i.e., a diamino-ether linker was protected as a t-Boc derivative prior to addition to the biotin activated ester. Any number of amine protecting groups can be utilized in this reaction. The primary purpose of the reaction method, as opposed to Example 2, is to be able to react one equivalent of diamino linker molecule with one equivalent of the biotin active ester. The reaction scheme is set forth below.
Reaction Step 1: Preparation of N-Boc-4,7,10-trioxatridecane-13-amine
[0095] To a solution of 151.40 g (687.25 mmol) of 4,7,10-trioxa-1,13-tridecaneamine in 700 mL CHCl3 was added 6.00 g (27.5 mmol) of di-tert-butyl dicarbonate in 100 mL CHCl3 with stirring at ambient temperature over 30 minutes. The mixture was stirred for 12 hours, washed with water ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com