Elimination of heterogeneous or mixed cell population in tumors
a heterogeneous or mixed cell population technology, applied in the field of tumor elimination or killing methods, can solve the problems of general compromise of the efficacy of antibody-drug conjugates, and achieve the effect of enhancing their stability or activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
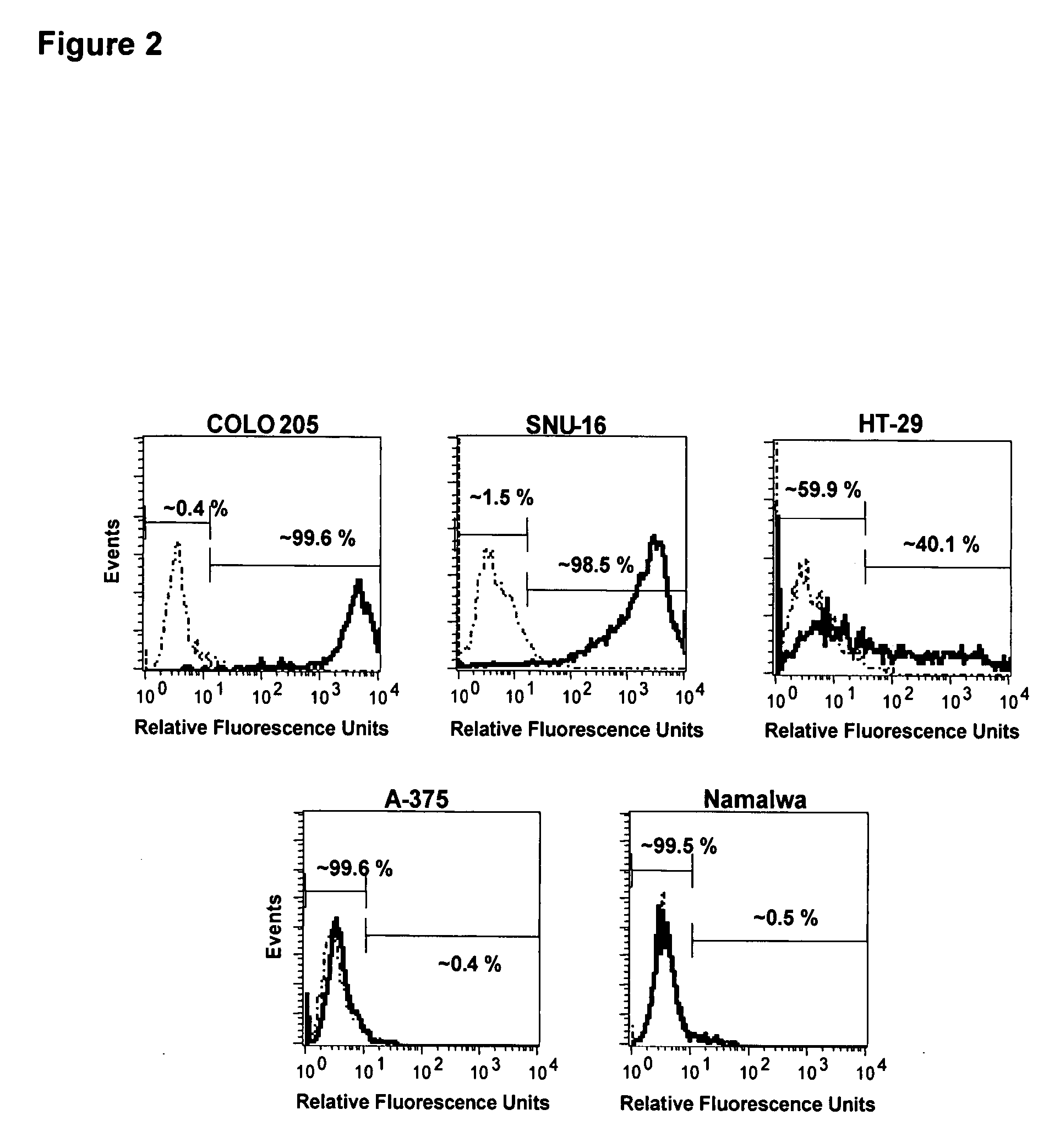
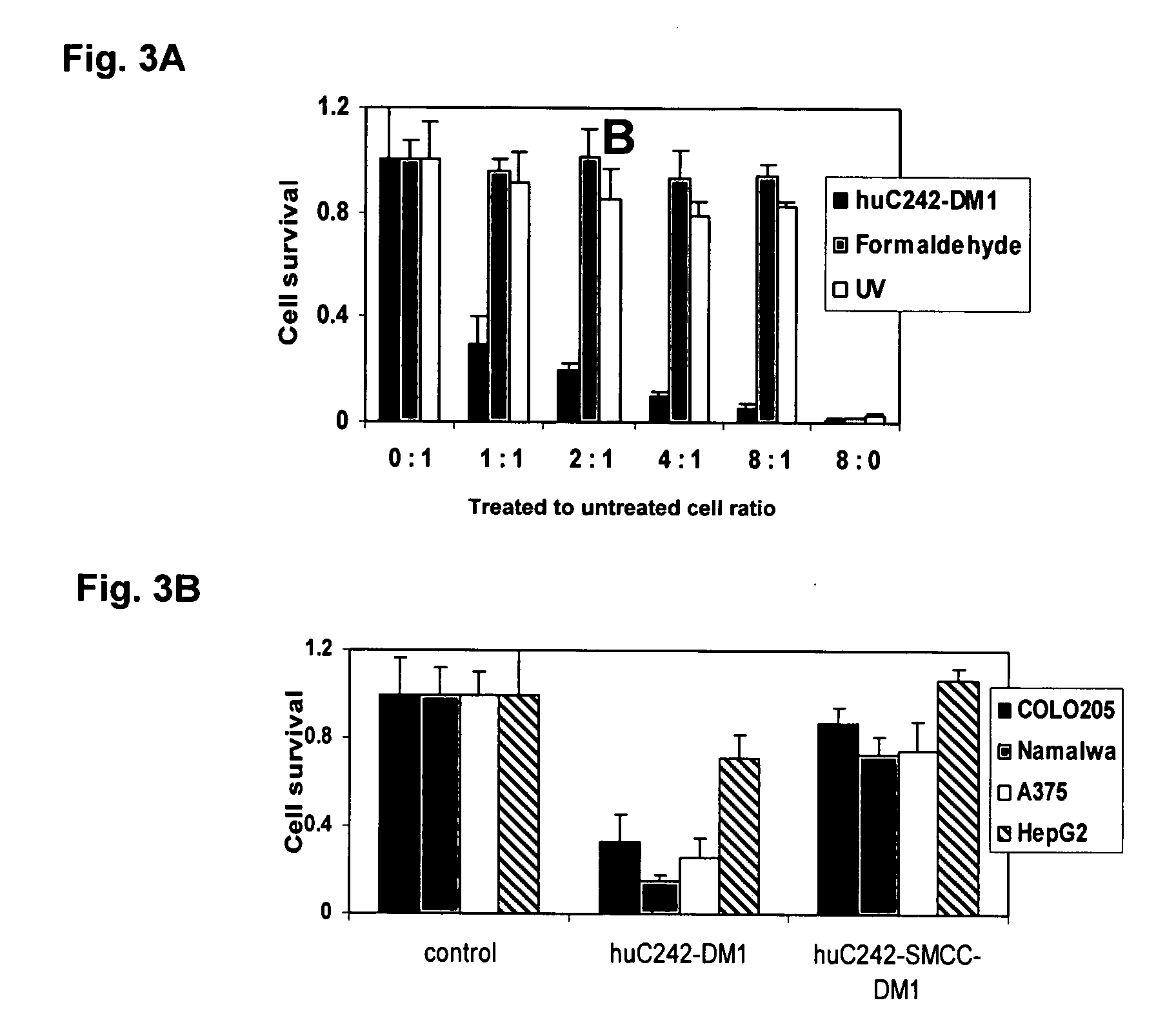
Bystander Effect of Antibody-Drug Conjugates
Materials and Methods
Immunoconjugates
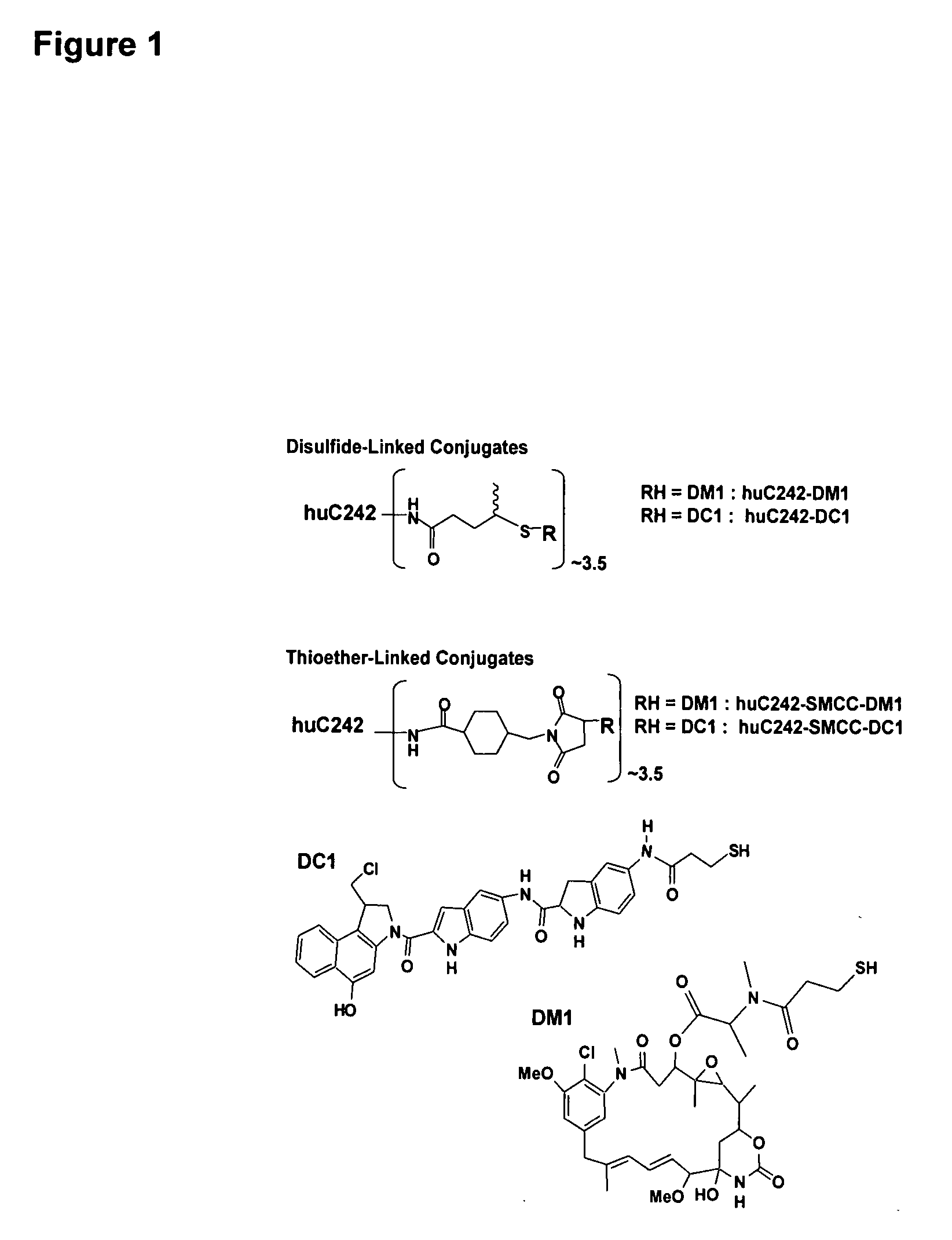
[0193] Preparation of immunoconjugates: The maytansinoid DM1 (N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine) was synthesized from the microbial fermentation product ansamitocin P-3 as described previously {Chari, R. V., et al., Cancer Res., 52, 127-31 (1992); U.S. Pat. No. 6,333,410}. The synthesis of the analogue of CC-1065, 5-[(3-mercapto-1-oxopropyl)amino]-bis-indolyl-(seco)-1,2,9,9a-tetrahydrocyclopropa[c]benz[e]indol-4-one, DC1, has been reported elsewhere {Chari, R. V., et al., Cancer Res. 55, 4079-84 (1995)}. The humanization of the C242 antibody (huC242) was done by the resurfacing method that has been described previously {Roguska M. A., et al., Proc Natl Acad Sci USA, 91, 969-73 (1994)}. Antibody-drug conjugates were prepared using N-succinimidyl-4-(2-pyridyldithio)pentanoate for disulfide linkage or N-succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) for thioether ...
example 2
Intracellular Activation of Antibody-Maytansinoid Coniugates
Abbreviations
[0214] BafA1, Bafilomycin A1
[0215] BSA, bovine serum albumin
[0216] FACS, fluorescence-activated cell sorter
[0217] HPLC, high pressure liquid chromatography
[0218] MTT, 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan
[0219] NEM, N-ethylmaleimide
[0220] SPDB, N-succinimidyl 4-(2-pyridyldithio)butyrate
[0221] SMCC, N-succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate
[0222] DM1, N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine
[0223] DM4, N2′-deacetyl-N2′-(4-mercapto-4-methyl-1-oxopentyl)-maytansine
[0224] DM1-SMe, N2′-deacetyl-N2′-[(3-methyldithio)-1-oxopropyl]-maytansine
[0225] DTT, dithiothreitol
Materials and Methods
[0226] RPMI 1640 and glutamine were from Cambrex Bioscience. Ultima Flo™ M scintillation fluid was from PerkinElmer life and analytical sciences. Gentamicin sulfate was from Gibco BRL. N-ethylmaleimide (NEM) and all other chemicals were obtained from Sigma. All antibodies use...
example 3
Synthesis of some Thiomethyl Maytansinoids (FIG. 17)
[0244] N2′-deacetyl-N2′-(3-methylthio-1-oxopropyl)-maytansine (S-Methyl-DM1, DM1Me): To a solution of N2′-deacetyl-N-2′(3-mercapto-1-oxopropyl)-maytansine (DM1, 30 mg, 0.041 mmol) in dimethylacetamide (0.25 mL) was added a solution of iodomethane (5.8 mg, 0.041 mmol) in 0.2 mL of dimethylacetamide under an argon atmosphere, with magnetic stirring. Diisopropylethylamine (5.0 mg, 0.041 mmol) was then added and the reaction was stirred for 3 hours. Purification by high pressure liquid chromatography using a reverse phase C 18 column and a gradient of deionized water and acetonitrile gave 20 mg (65% yield) of the desired product DM1Me. Mass spectrum: found 774.5 (M+Na+), calculated 774.3 (M+Na+); 1H NMR (CDCl3) δ 0.84 (3H, s), 1.33 (3H, d, J=5 Hz), 1.35 (3H, d, J=5 Hz), 1.60 (3H, s), 1.68 (3H, s), 2.05 (3H, s) 2.22 (1H, dd, J=3 Hz, 14 Hz, 2.60-2.82 (2H, m), 2.88 (3H, s), 3.08-3.20 (2H, m), 3.25 (3H, s), 3.39 (3H, s), 3.55 (1H, d, J=9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com