Transdermal Method and Patch for Nausea
a technology of transdermal devices and patches, applied in the field of transdermal devices and methods for the treatment can solve the problems of nausea and vomiting, difficulty in antiemetic agents, and side effects of treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Adhesive Mixture and Transdermal Delivery Device
[0071]
TABLE 1CompositionFormulation AFormulation BPatch Size15.0 cm215.0 cm2Estimated Target Daily1.2 mg1.2 mgDoseDry %Dry %Styrene-butadiene rubber44.04—pressure sensitive adhesiveAcrylate-vinylacetate—43.8pressure sensitive adhesiveIsopropyl Myristate3.014.07Granisetron Base3.053.05Polyester Release liner35.835.8Polyester Backing13.313.3
Components
[0072] Formulation A and Formulation B were prepared using the amounts of each component as shown in Table 1 above.
[0073] The styrene-butadiene rubber pressure sensitive adhesive used in the examples herein was DURO-TAK®87-6173 adhesive, available from National Starch and Chemical in Bridgewater, N.J. The acrylate-vinylacetate pressure sensitive adhesive used in the examples herein was DURO-TAK®87-2516 adhesive, available from National Starch and Chemical in Bridgewater, N.J. The isopropyl myristate used in the examples herein was of NF grade. The polyester release liner u...
example 2
Test for Flux of Granisetron from the Transdermal Delivery Device
Procedure
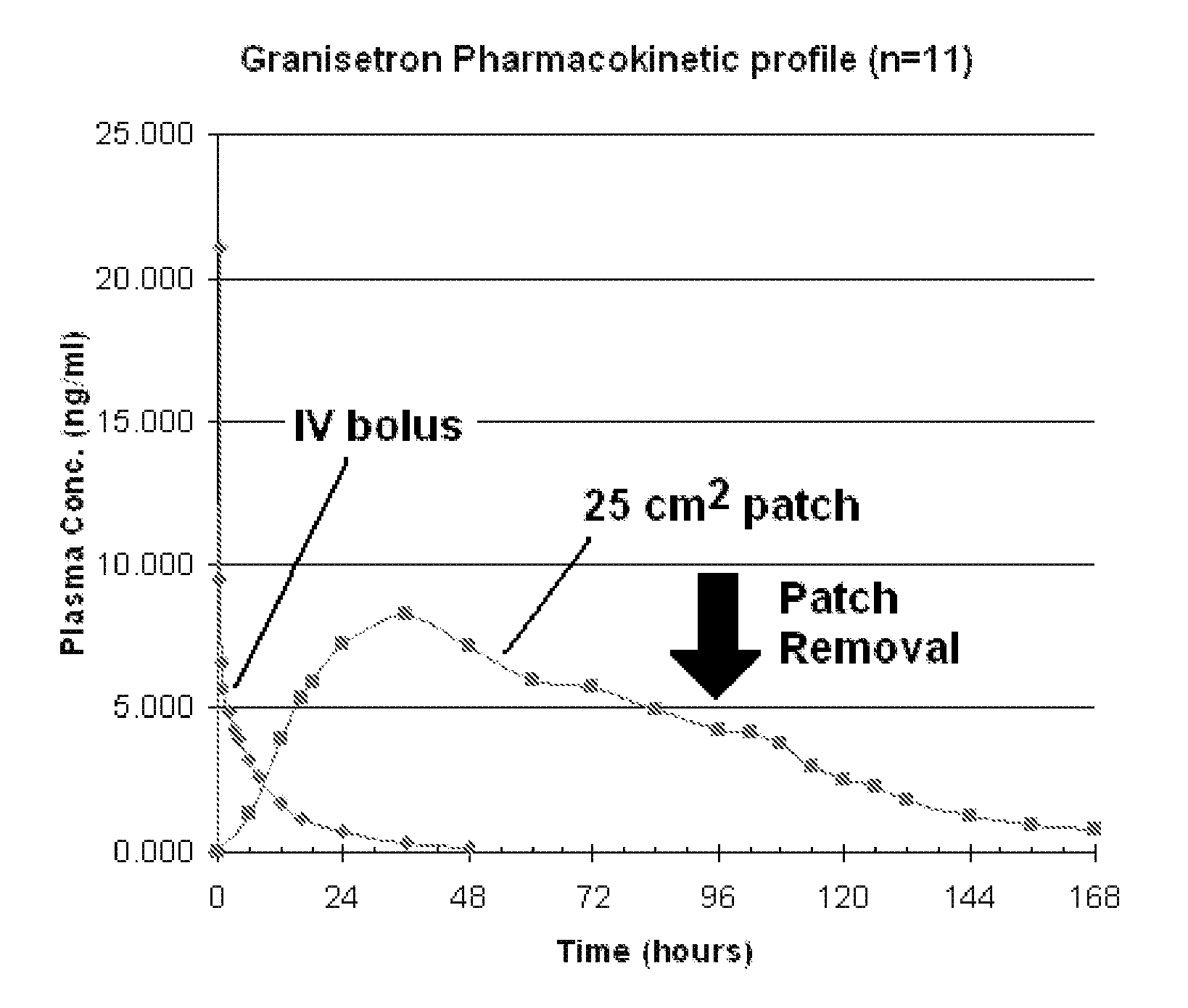
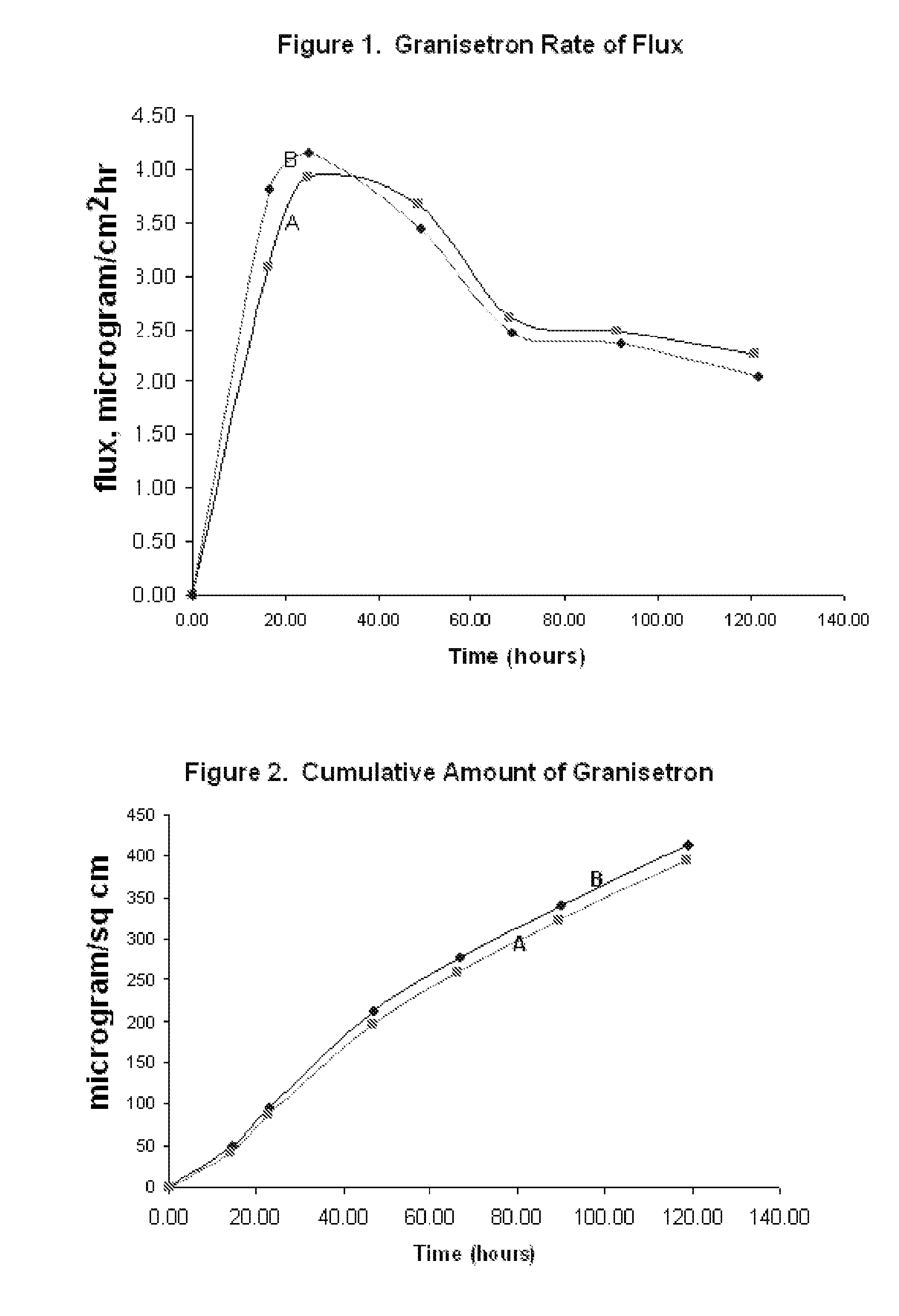
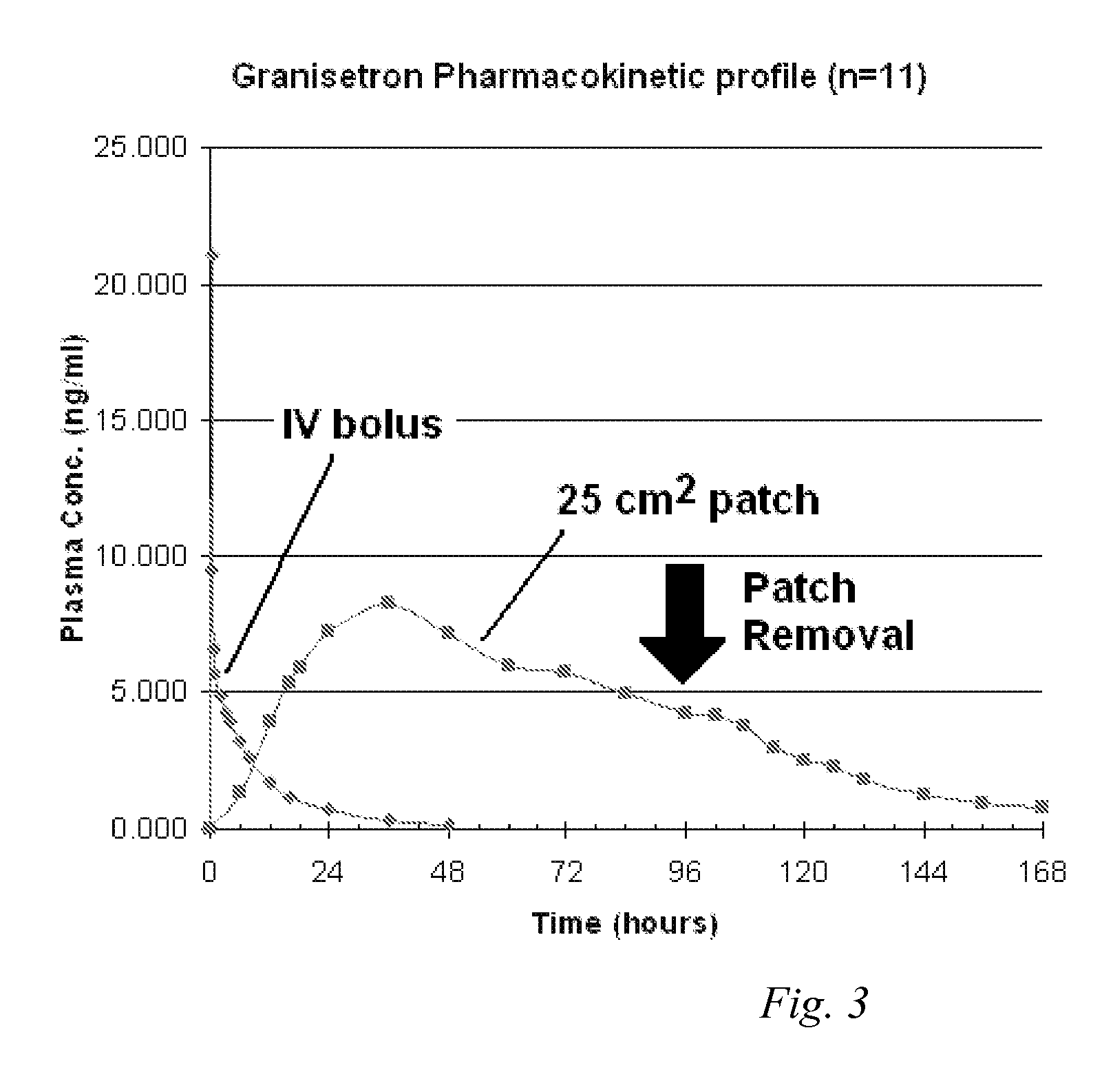
[0077] Heat-separated human cadaver skin was cut to the desired size and mounted on a Franz diffusion cell. The release liner was peeled away from a patch made according to Formulation B as described in EXAMPLE 1 above. The patch was placed on the skin and the patch and skin were clamped together. Receptor solution was added to the diffusion cell, and the assembly was maintained at 32° C. Aliquots of the receptor solution were taken at periodic time points (24 hours, 48 hours, 72 hours, 96 hours, and 120 hours). The concentration of the granisetron in the receptor solution was measured at each time point, and the flux rate from examples A and B was calculated. The resulting data is illustrated in FIG. 1. Cumulative delivery of granisetron over the indicated time was likewise calculated from the concentration of granisetron in the receptor solution at each time point and is illustrated in FIG. 2.
example 3
Stability of Granisetron in Illustrative Examples
[0078]
TABLE 2Formulation CFormulation DCompositionDry %Dry %Styrene-butadiene rubber49.88% —pressure sensitive adhesiveAcrylate-vinylacetate43.77% pressure sensitive adhesiveIsopropyl Myristate—5.09%Granisetron Base*1.02%2.04%Polyester Release liner35.8%35.8%Polyester Backing13.3%13.3%
[0079] Patches were made according to the procedure described in Example 1 above using the formulations shown in Table 2. The patches were then tested for granisetron stability using the method described below.
[0080] Samples of the patches are stored at 50° C. for up to 2 months. Stability of the product is assessed by testing periodically for granisetron content and the total amount of impurities using high performance liquid chromatography. The results are shown below in Table 3.
TABLE 3Total impuritiesGranisetron Potency(% of granisetron)(% w / w)Formu-TimeFormulation CFormulation Dlation CFormulation DTo99.999.50.050.251 month at99.899.20.120.4150° ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com