Polyolefin functional at one end
a functional polyolefin and polyolefin technology, applied in the field of single-chainend functionalized polyolefins, can solve the problems of high chemical stability of polyolefins, difficulty in controlling the structure of olefin chain moieties in the resultant polymers, and damage to the original physical properties of polyolefins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
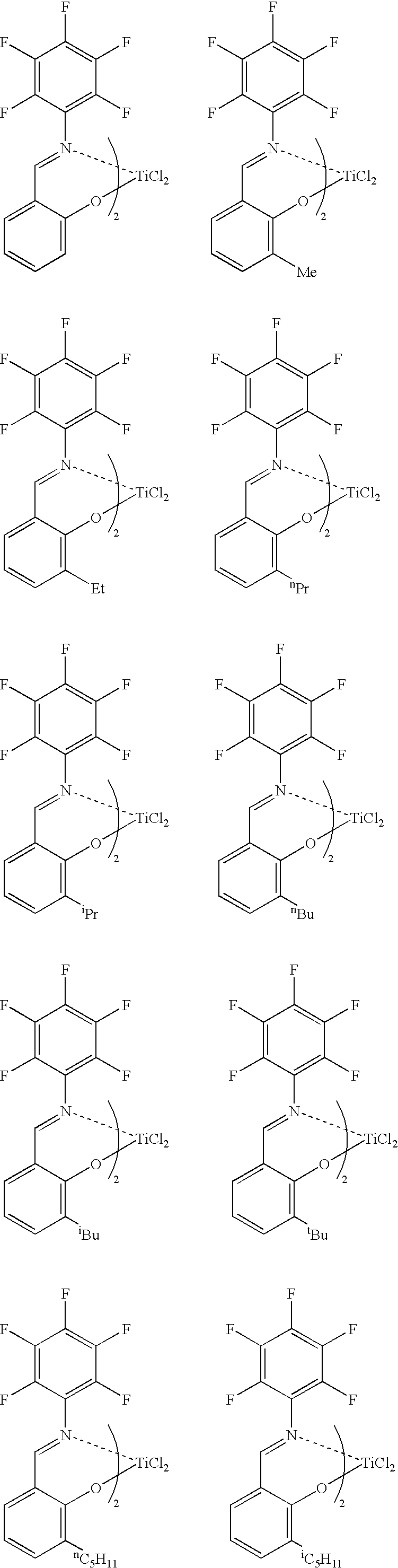
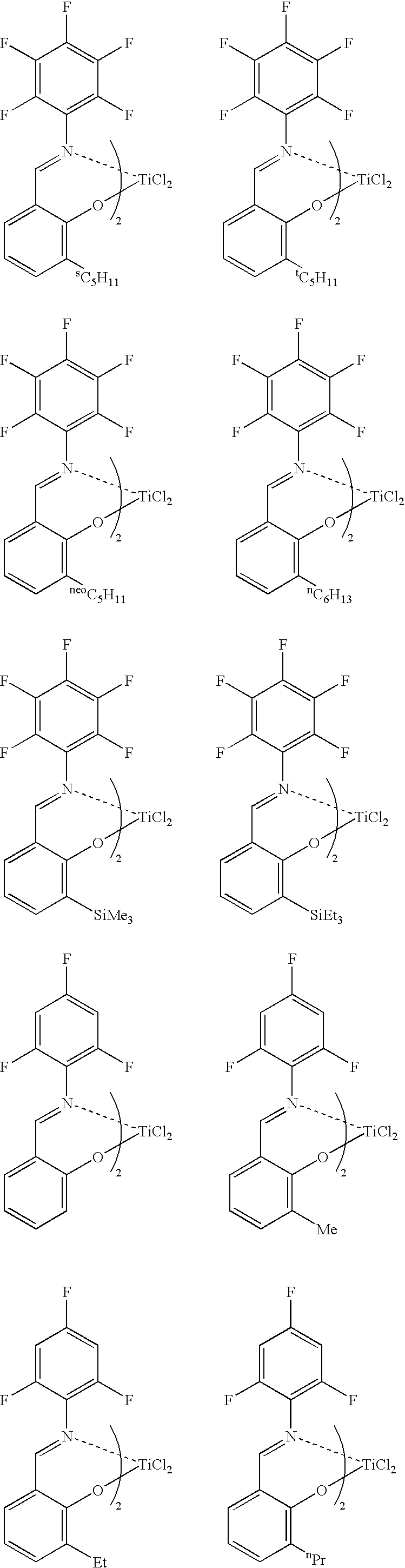
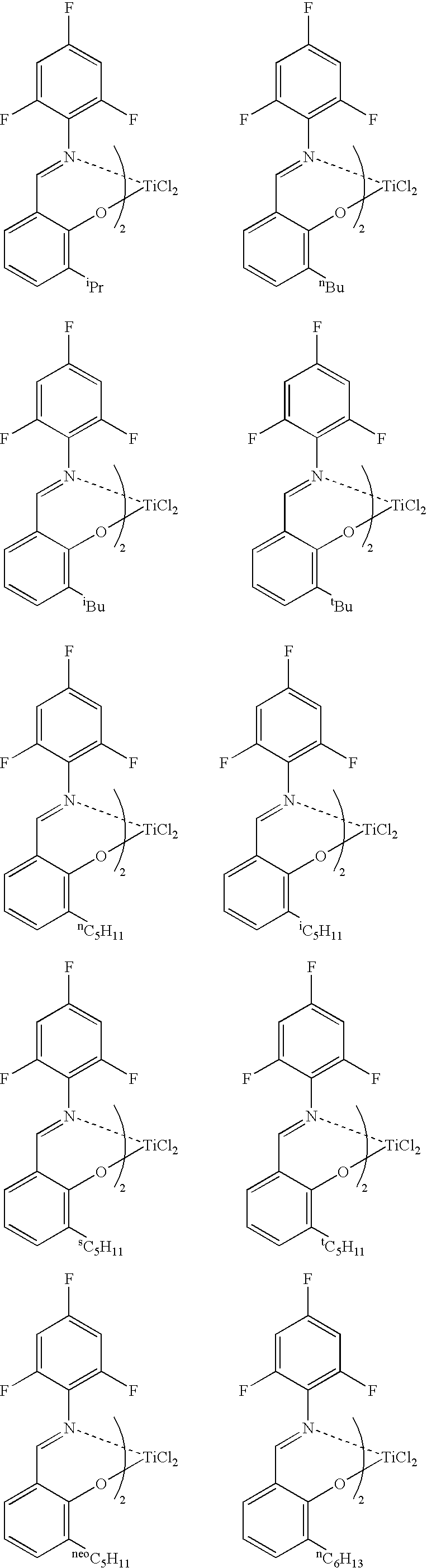
[0042] Into a glass reactor having an internal volume of 500 mL and purged sufficiently with nitrogen were charged 250 mL of toluene and 15.2 mmol of methylaluminoxane, the amount being an amount in terms of aluminum atoms therein. Thereto was added a solution of 21.3 mg (0.136 mmol) of Me2AlO—(CH2)4CH═CH2 in toluene. Thereto was added a solution of 88.9 mg (containing diethyl ether, 0.101 mmol) of a titanium complex, bis[N-(3-t-butylsalicylidene)-2,3,4,5,6-pentafluoroanilinate]titanium dichloride intoluene, and then the components were caused to react at 27° C. for 15 minutes. Thereafter, the reaction solution was cooled to 0° C. Thereafter, a mixed gas of ethylene and nitrogen (gas flow rate: ethylene, 5 L / h; and nitrogen, 50 L / h), the pressure of which was a normal pressure, was blown from the bottom of the reactor to the inside thereof so as to cause the components to react at 0° C. for 5 minutes. Thereafter, the supply of ethylene was stopped and methanol was added thereto, the...
example 2
[0043] Into a glass reactor having an internal volume of 500 mL and purged sufficiently with nitrogen were charged 250 mL of toluene and 10.0 mmol of methylaluminoxane, the amount being an amount in terms of aluminum atoms therein. The reaction solution was cooled to 0° C., and then thereto was added a solution of 10.7 mg (0.0685 mmol) of Me2AlO—(CH2)4CH═CH2 in toluene. Thereto was added a solution of 58.4 mg (containing the weight of diethyl ether, 0.0666 mmol) of a titanium complex, bis[N-(3-t-butylsalicylidene)-2,3,4,5,6-pentafluoroanilinate] titanium dichloride in toluene, and then the components were caused to react at 0° C. for 30 minutes. Thereafter, propylene (gas flow rate: 100 L / h), the pressure of which was a normal pressure, was blown from the bottom of the reactor to the inside thereof so as to cause the components to react at 0° C. for 105 minutes. Thereafter, the supply of propylene was stopped, and methanol was added thereto, thereby terminating the polymerization. A...
example 3
[0044] Propylene was polymerized under the same conditions as in Example 2 except that Me2AlO—(CH2)9CH═CH2 was used instead of Me2AlO—(CH2)4CH═CH2. The polymerization activity per mmol of titanium was 3.03 g, the number-average molecular weight (Mn) of the polymer was 8,200, and the ratio of the weight-average molecular weight (Mw) to the number-average molecular weight (Mn), (Mw / Mn), was 1.09. In the 1H NMR spectrum (FT, 270 MHz in C2D2Cl4, at 120° C.) of this polymer, a triplet corresponding to a methylene group adjacent to an OH group made its appearance near 3.64 ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com