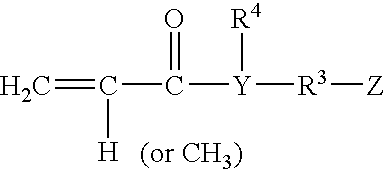
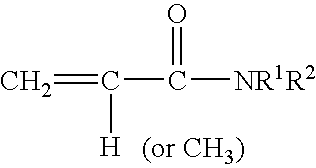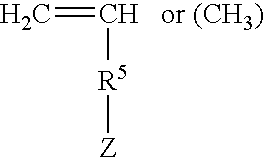Rapid drying lacquers containing triblock copolymer for rheology control
a technology of triblock copolymer and rheology control, which is applied in the direction of coatings, etc., can solve the problems of adverse effects on the appearance of the basecoat, no one can meet the rapid drying times that are desired, and the metallic flake control and metallic appearance (or downflop) of the basecoat will suffer, so as to achieve the effect of improving the film properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of MAA / HEMA / ETEGMA Macromonomer, 60 / 20 / 20% by Weight
[0117] This example illustrates the preparation of a macromonomer with carboxyl groups, primary hydroxyl groups, and polyethylene oxide groups that are capable of forming hydrogen bonds and can be used to form the A block (outer block) of a triblock copolymer of this invention. A 5-liter flask was equipped with a thermometer, stirrer, additional funnels, heating mantel, reflux condenser and a means of maintaining a nitrogen blanket over the reactants. The flask was held under nitrogen positive pressure and the following ingredients were employed.
Weight (gram)Portion 1Methyl ethyl ketone850.0Isopropanol990.0Portion 2Diaquabis(borondifluorodiphenyl0.48glyoximato)cobaltate (II), Co(DPG-BF2)Acetone106.4Portion 32,2′-Azobis(methylbutyronitrile) (Vazo ®21.667 by DuPont Co., Wilmington, DE)Methyl ethyl ketone260.0Portion 4Methacrylic acid (MAA)720.02-Hydroxyethyl methacrylate (HEMA)240.0Ethoxy triethyleneglycol methacrylate...
example 2
Preparation of an AB Diblock Macromonomer BMA / MMA / / MAA / HEMA / ETEGMA, 45 / 30 / 15 / 5 / 5% by Weight
[0120] This example shows the preparation of a diblock macromonomer where the B block (center block) has no specific functional groups and the A block (one of the terminal block) contains carboxyl groups, primary hydroxyl groups, and polyethylene oxide groups from the macromonomer prepared above.
[0121] A 5-liter flask was equipped as in Example 1. The flask was held under nitrogen positive pressure and the following ingredients were employed.
Weight (gram)Portion 1Macromonomer of Example 11257.15Isopropanol614.8Portion 2Methyl methacrylate (MMA)528.0Butyl methacrylate (BMA)792.0Portion 3t-Butyl peroctoate (Elf Atochem North28.0America, Inc., Philadelphia, PA)Ethyl acetate300.0Total3519.95
[0122] Portion 1 mixture was charged to the flask and the mixture was heated to reflux temperature and refluxed for about 10 minutes. Portion 2 was added over 3 hours and Portion 3 was simultaneously added ...
example 3
Preparation of an ABA′ Triblock Copolymer
[0124] This example shows the preparation of an ABA′ triblock copolymer of this invention containing carboxyl groups, and primary hydroxyl groups on both the A and A′ blocks, no specific functional groups on the center B block, specifically methyl methacrylate-co-butyl methacrylate-co-2-hydroxyethyl methacrylate-co-methacrylic acid-b-butyl methacrylate-co-methyl methacrylate-b-methacrylic acid-co-hydroxyethyl methacrylate-co-ethoxytriethyleneglycol methacrylate, 32 / 22 / 7 / 4 / / 15.75 / 10.51 / 5.25 / 1.75 / 1.75% by weight, from a macromonomer prepared above.
[0125] A 12-liter flask was equipped as in Example 1. The flask was held under nitrogen positive pressure and the following ingredients were employed.
Weight (gram)Portion 1Macromonomer of Example 22350.0Ethyl acetate960.0Portion 2Methyl methacrlate (MMA)1075.0Butyl methacrylate (BMA)740.02-Hydroxyethyl methacrylate (HEMA)236.0Methacrylic acid135.0Portion 3t-Butyl peroctoate (Elf Atochem North45.0A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com