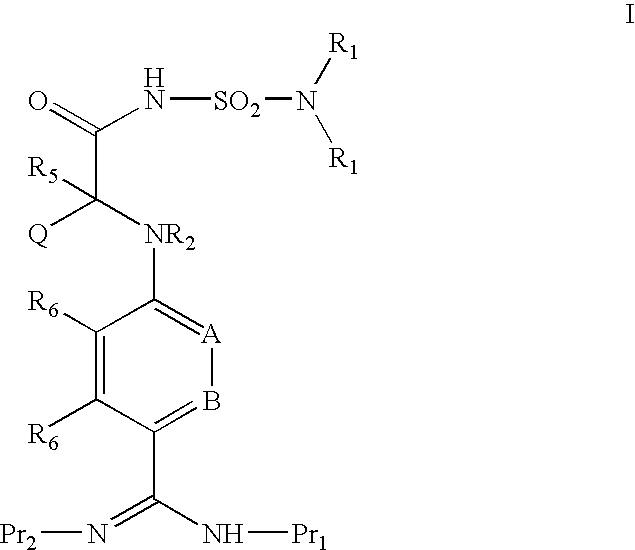
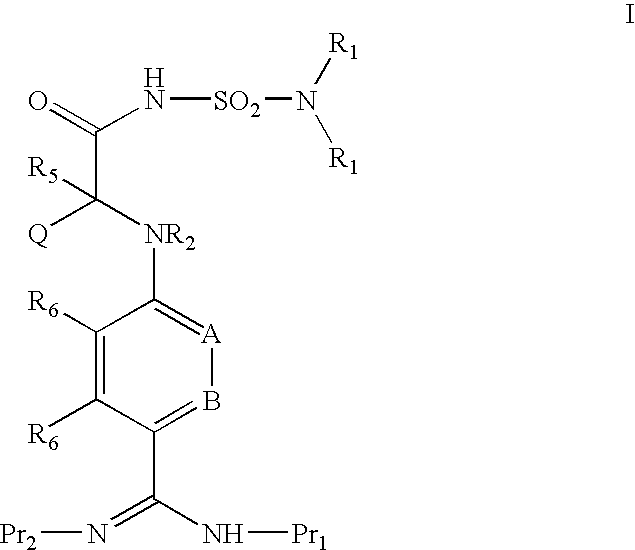
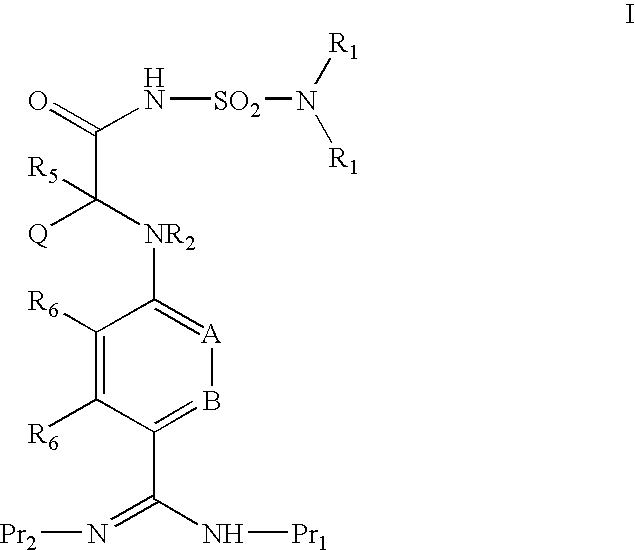
ACYLSULFAMIDE INHIBITORS OF FACTOR VIIa
a technology of acylsulfamide and inhibitors, applied in the direction of biocide, drug composition, extracellular fluid disorder, etc., can solve the problems of pulmonary embolism, reduced blood flow to the affected area, and reduced blood flow, so as to improve the pharmacokinetic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0199]
[0200] 4-Benzyloxy-5-methoxy-2-nitrobenzaldehyde (12.2 g 42 mmoles) and 4-aminobenzonitrile (5 g, 42 mmoles) were dissolved in methanol (165 ml) and stirred for two hours and then heated to 60° C. for 30 minutes. The reaction was allowed to cool to room temperature and benzyl isonitrile (5 g. 42 mmoles) added. The reaction was cooled to 0° C. and boron trifluoroetherate (16 ml, 126 mmoles) added dropwise over five minutes. The reaction was stirred at 0° C. for 20 minutes and then allowed to come to room temperature and then stirred at ambient temperature for two hours. Water (4 ml) was added and the mixture stirred at room temperature overnight. A yellow precipitate was evident the next morning and the solid filtered off. The solid was washed with methanol and air dried to yield 8 grams of the desired product. The solvent from the filtrate was removed in vacuo and replaced with ethyl acetate. The solution was washed with water and saturated sodium bicarbonate, dried over anhyd...
example 2
[0201]
[0202] The methyl ester of the acid above (920 mg 2.85 mmoles) was suspended in 3 / 1 THF / water (40 ml) and cooled to 0° C. The solution was treated with 1 N LiOH (7.1 ml, 7.1 mmoles) and allowed to stir overnight. The reaction was acidified with trifluoroacetic acid until pH=4.0 was obtained. The solvent was removed in vacuo and the crude material purified by flash chromatography (ethyl acetate with 0.5% acetic acid) to yield Ig of carboxylic acid.
example 3
[0203]
[0204] 4,5-diethoxy-2-nitrobenzaldehyde (55.5 gm, 206 mmoles) and 4-aminobenzonitrile (23 g, 195 mmoles) were dissolved in methanol (700 ml) and stirred at 60° C. for 2 hours. The reaction was allowed to cool to 0° C. and tosylmethylisonitrile (45 g. 230 mmoles) added. Boron trifluoroetherate (78 ml, 620 mmoles) was added dropwise over 10 minutes. The reaction was stirred at 0° C. for 30 minutes, allowed to come to room temperature and then stirred at ambient temperature for 1.5 hours. Water (18 ml) was added and the mixture stirred at room temperature overnight. The following day the methanol was removed in vacuo and the residue taken up in ethyl acetate. The organic layer was washed with water and then dried over anhydrous sodium sulfate. The sodium sulfate was filtered off and the ethyl acetate removed in vacuo. The crude material was submitted to flash chromatography (hexanes:ethyl acetate, 2:1 then 1:1) to yield 46 g of the desired product (4-ethoxy-5-ethoxy-2-nitro-pheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com