Apparatus and method for sequencing a nucleic acid
a nucleic acid and apparatus technology, applied in the field of apparatus and methods for determining the sequence of nucleic acids, can solve the problems of laborious processing of the large number of samples required for electrophoretic-based nucleic acid based analysis, less desirable electrophoretic-based separation analysis, and large amount of input dna
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Anchor Primers Linked to a Cavitated Terminus Fiber Optic Array
[0262] The termini of a thin wafer fiber optic array are cavitated by inserting the termini into acid as described by Healey et al., Anal Chem. 69: 2213-2216 (1997).
[0263] A thin layer of a photoactivatable biotin analog is dried onto the cavitated surface as described in Hengsakul and Cass (Bioconjugate Chem. 7: 249-254, 1996) and exposed to white light through a mask to create defined pads, or areas of active biotin. Next, avidin is added and allowed to bind to the biotin. Biotinylated oligonucleotides are then added. The avidin has free biotin binding sites that can anchor biotinylated oligonucleotides through a biotin-avidin-biotin link.
[0264] The pads are approximately 10 μm on a side with a 100 μm spacing. Oligonucleotides are added so that approximately 37% of the pads include one anchored primer. On a 1 cm2 surface are deposited 10,000 pads, yielding approximately 3700 pads with a single anchor...
example 2
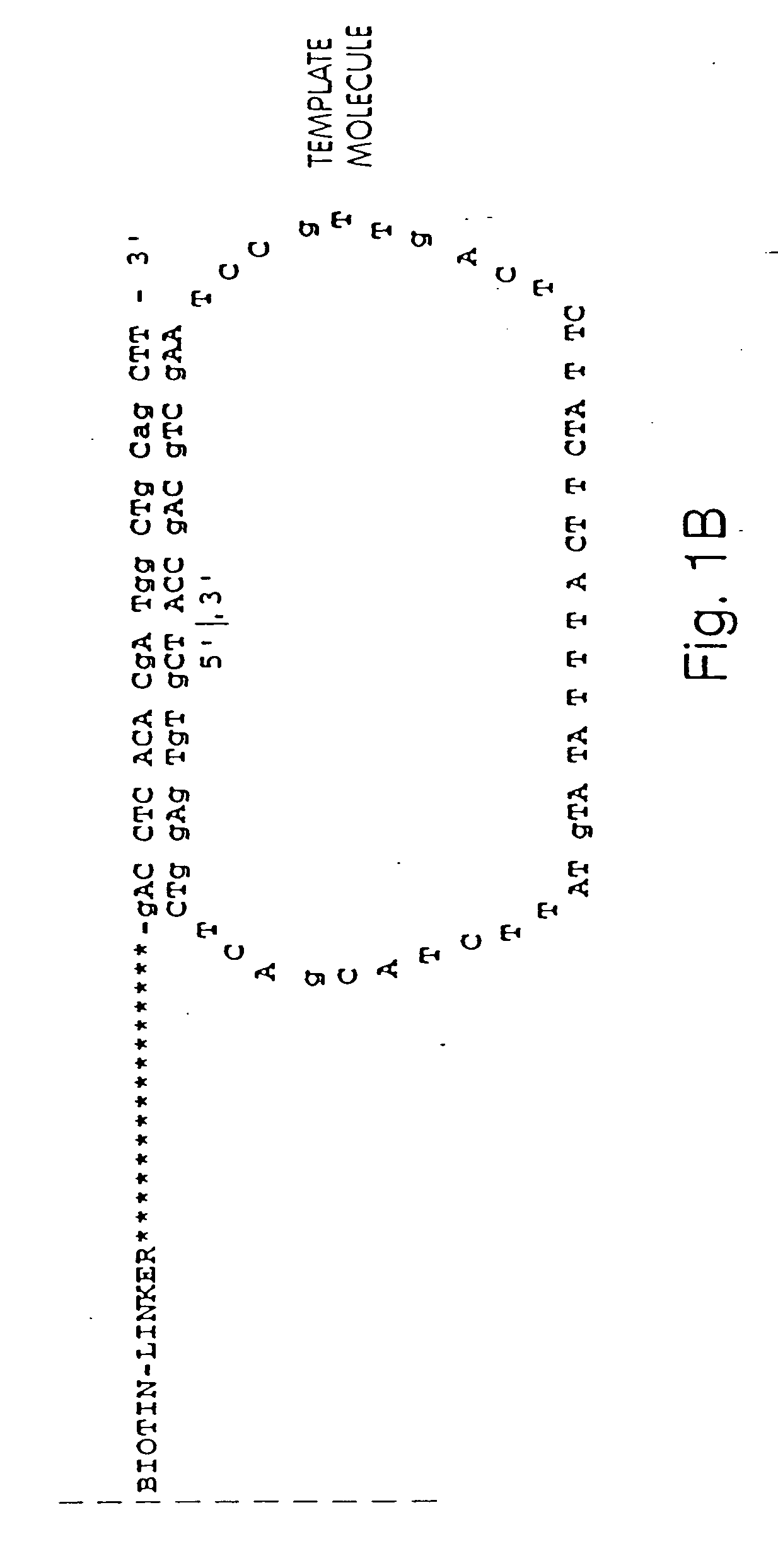
Annealing and Amplification of Members of a Circular Nucleic Acid Library
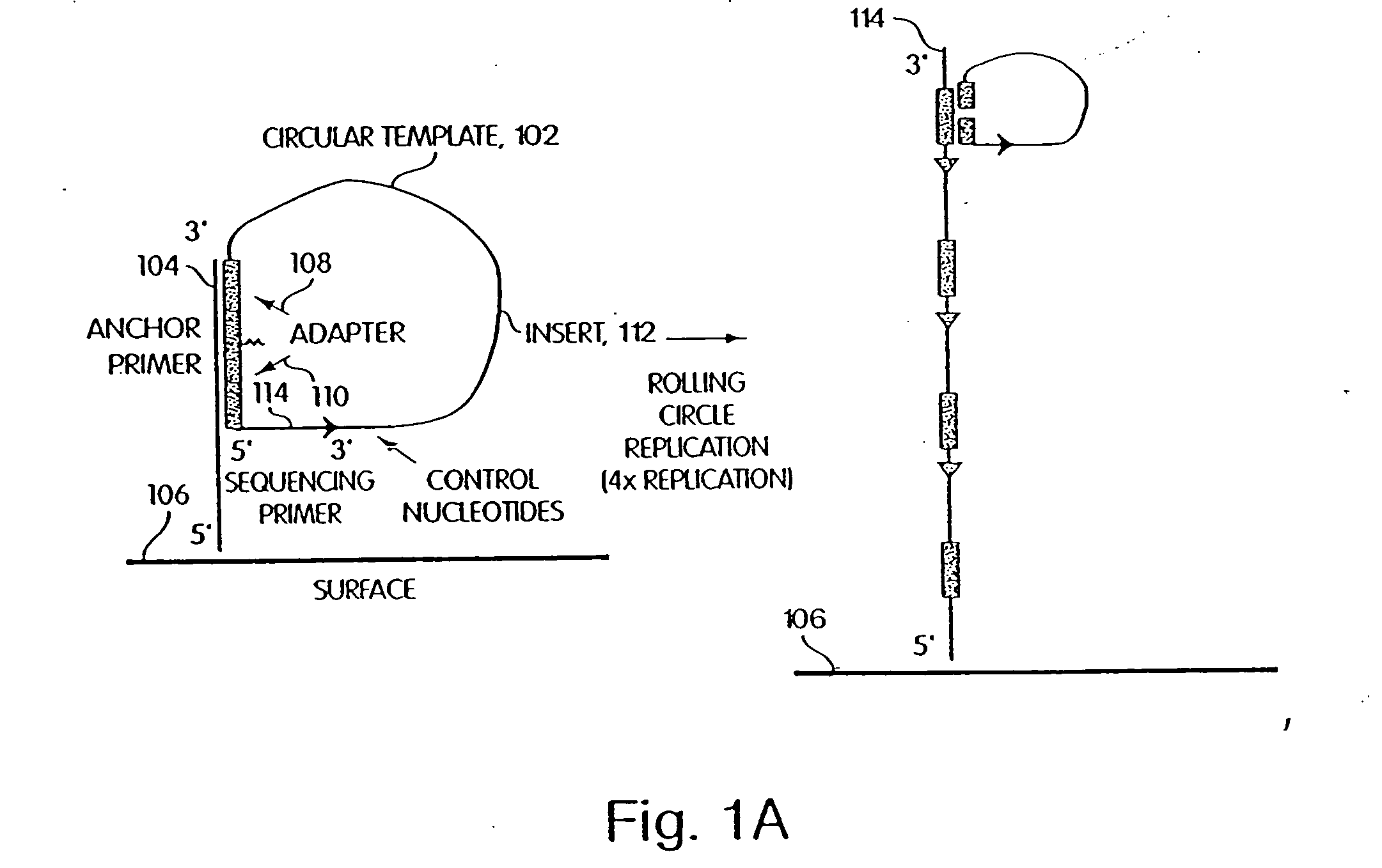
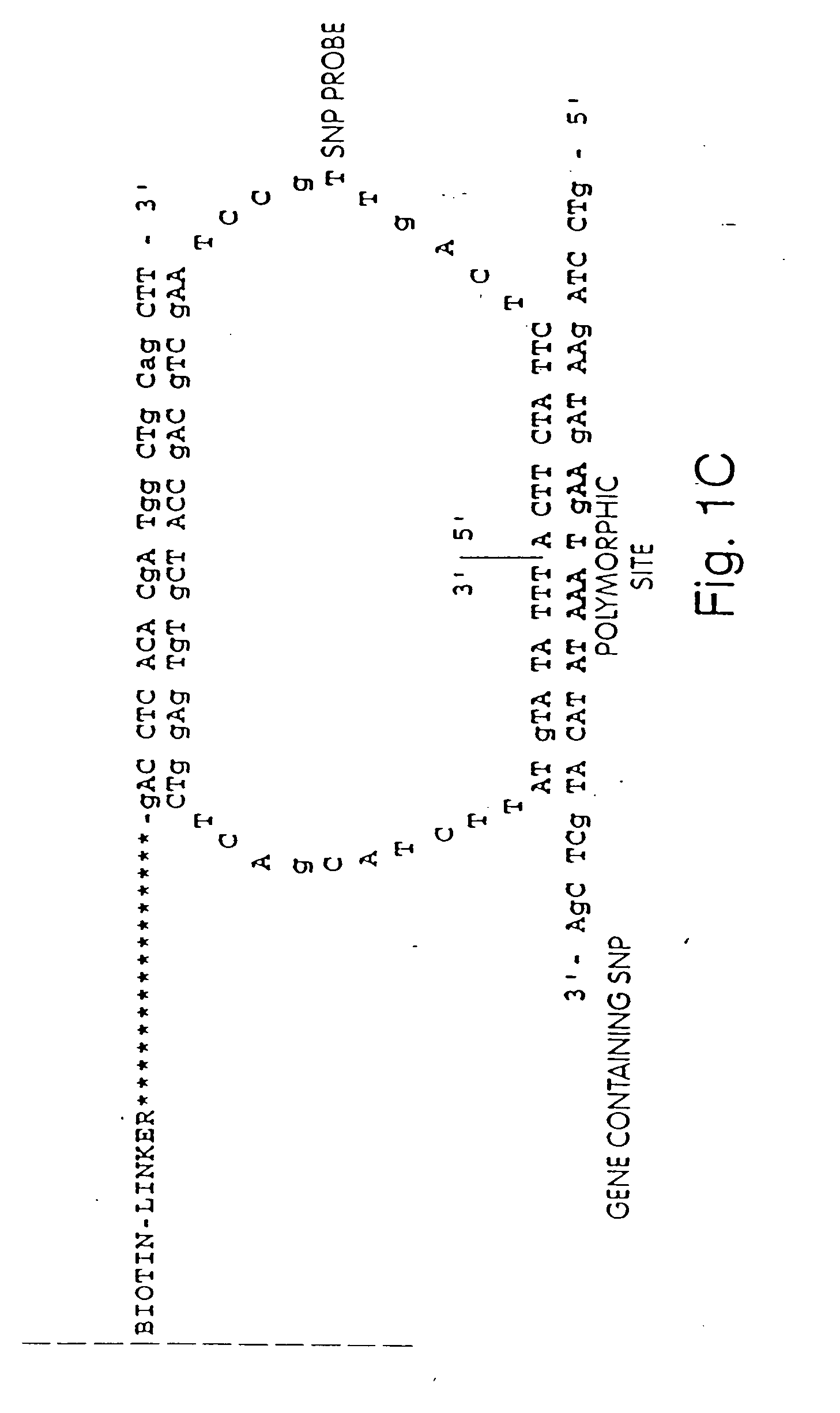
[0265] A library of open circle library templates is prepared from a population of nucleic acids suspected of containing a single nucleotide polymorphism on a 70 bp Sau3A1-MspI fragment. The templates include adapters that are complementary to the anchor primer, a region complementary to a sequencing primer, and an insert sequence that is to be characterized. The library is generated using Sau3A1 and MspI to digest the genomic DNA. Inserts approximately 65-75 nucleotides are selected and ligated to adapter oligonucleotides 12 nucleotides in length. The adapter oligonucleotides have sequences complementary to sequences to an anchor primers linked to a substrate surface as described in Example 1.
[0266] The library is annealed to the array of anchor primers. A DNA polymerase is added, along with dNTPs, and rolling circle replication is used to extend the anchor primer. The result is a single DNA strand, still an...
example 3
Sequence Analysis of Nucleic Acid Linked to the Terminus of a Fiber Optic Substrate
[0267] The fiber optic array wafer containing amplified nucleic acids as described in Example 2 is placed in a perfusion chamber and attached to a bundle of fiber optic arrays, which are themselves linked to a 16 million pixel CCD camera. A sequencing primer is delivered into the perfusion chamber and allowed to anneal to the amplified sequences. Then sulfurylase, apyrase, and luciferase are attached to the cavitated substrate using biotin-avidin.
[0268] The sequencing primer primes DNA synthesis extending into the insert suspected of having a polymorphism, as shown in FIG. 1. The sequencing primer is first extended by delivering into the perfusion chamber, in succession, a wash solution, a DNA polymerase, and one of dTTP, dGTP, dCTP, or α thio dATP (a dATP analog). The sulfurylase, luciferase, and apyrase, attached to the termini convert any PPi liberated as part of the sequencing reaction to detect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com