Method for Activating Surface of Metal Member
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
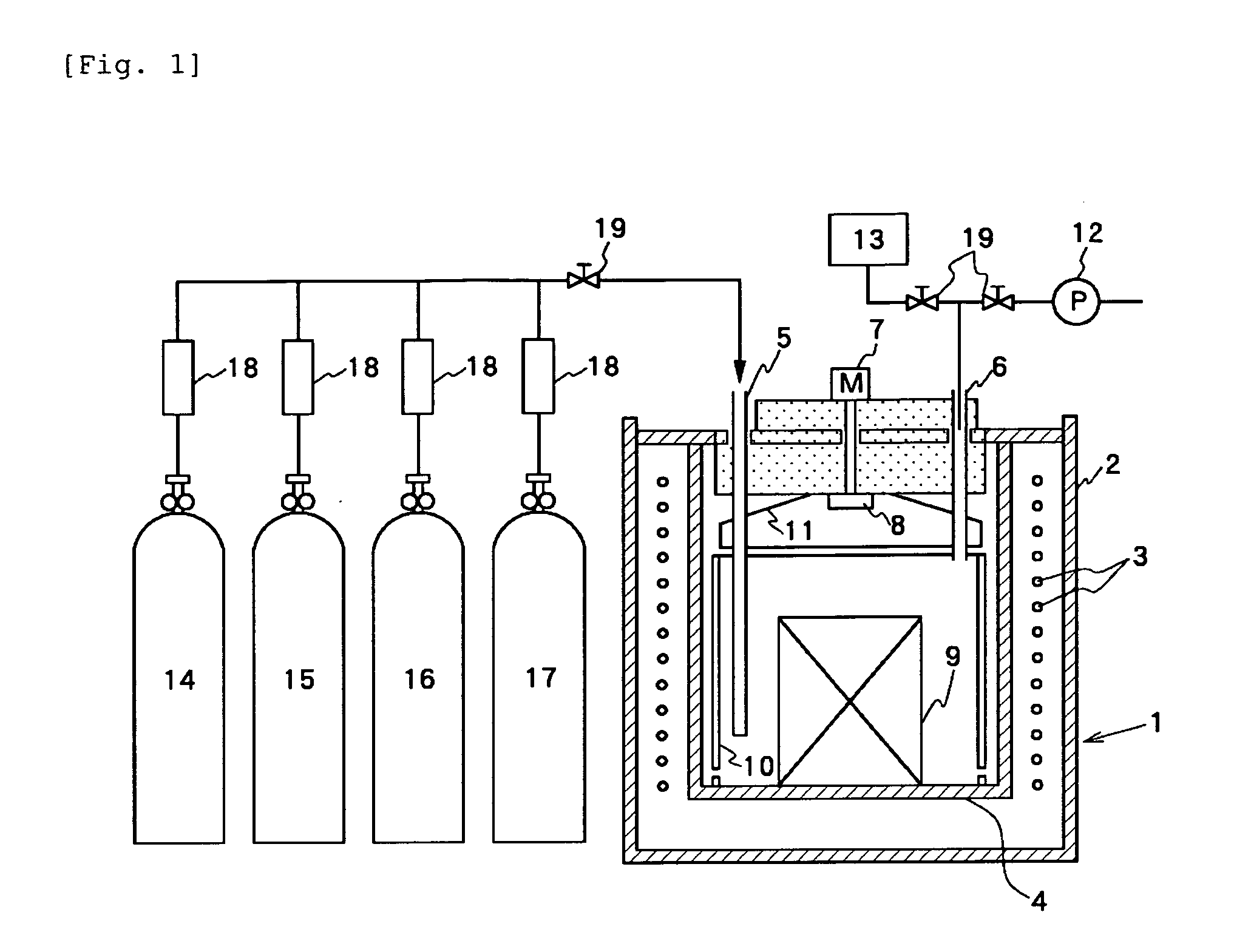
[0051] Using the SUS310S Muffle furnace of 100-L internal capacity shown in FIG. 1, SUS304 plates were set in the furnace, NH3 gas and N2 gas were fed at flow rates of 200 L / H, respectively, and the furnace atmosphere was heated from room temperature to 550° C. in 75 minutes. At the time point that the atmosphere temperature had reached 100° C. in the course of the heating (at the 18th minute after the initiation of the heating), an injection of acetylene gas was initiated at 2 L / hr. After heated to 550° C., the atmosphere temperature was maintained for 2 hours. At that time point, the injection of acetylene gas was terminated and instead, NH3 gas and N2 gas were then fed at 550° C. for 4 hours to allow nitriding to proceed. Subsequently, the heating was stopped and N2 gas alone was continuously fed to cool down the furnace. When the atmosphere temperature had dropped to 100° C. or lower, the specimens were taken out of the furnace.
[0052] Effluent gas from the furnace was branched ...
example 2
[0053] SUS304 plates were set in the Muffle furnace employed in Example 1, NH3 gas and N2 gas were fed at flow rates of 200 L / H, respectively, and the furnace atmosphere was heated from room temperature to 550° C. in 75 minutes. At the time point that the atmosphere temperature had reached 100° C. in the course of the heating (at the 18th minute after the initiation of the heating), an injection of propane gas was tinitiated at 5 L / hr. After heated to 550° C., the atmosphere temperature was maintained for 2 hours. At that time point, the injection of propane gas was terminated and instead, NH3 gas and N2 gas were then fed at 550° C. for 4 hours to allow nitriding to proceed. Subsequently, the heating was stopped and N2 gas alone was continuously fed to cool down the furnace. When the atmosphere temperature had dropped to 100° C, or lower, the specimens were taken out of the furnace.
[0054] Effluent gas from the furnace was branched off to have a portion of the effluent gas absorbed ...
example 3
[0055] SUS304 plates were set in the Muffle furnace employed in Example 1, NH3 gas and N2 gas were fed at flow rates of 200 L / H, respectively, and the furnace atmosphere was heated from room temperature to 550° C. in 75 minutes. At the time point that the atmosphere temperature had reached 100° C. in the course of the heating (at the 18th minute after the initiation of the heating), an injection of CO gas was initiated at 5 L / hr. After heated to 550° C., the atmosphere temperature was maintained for 2 hours. At that time point, the injection of CO gas was terminated and instead, NH3 gas and N2 gas were then fed for 4 hours to allow nitriding to proceed. Subsequently, the heating was stopped and N2 gas alone was continuously fed at 550° C. to cool down the furnace. When the atmosphere temperature had dropped to 100° C. or lower, the specimens were taken out of the furnace.
[0056] Effluent gas from the furnace was branched off to have a portion of the effluent gas absorbed in a 2 wt. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Dew point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com