Method of leaching copper sulfide ores containing chalcopyrite
a technology of copper sulfide ores and leaching methods, which is applied in the direction of biological water/sewage treatment, water/sludge/sewage treatment, chemistry apparatus and processes, etc., can solve the problems of limited target ores used for hydrometallurgy, copper oxide ore reserves that can be used, and leaching takes several years to achieve the effect of efficient and cost-effectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 and 2
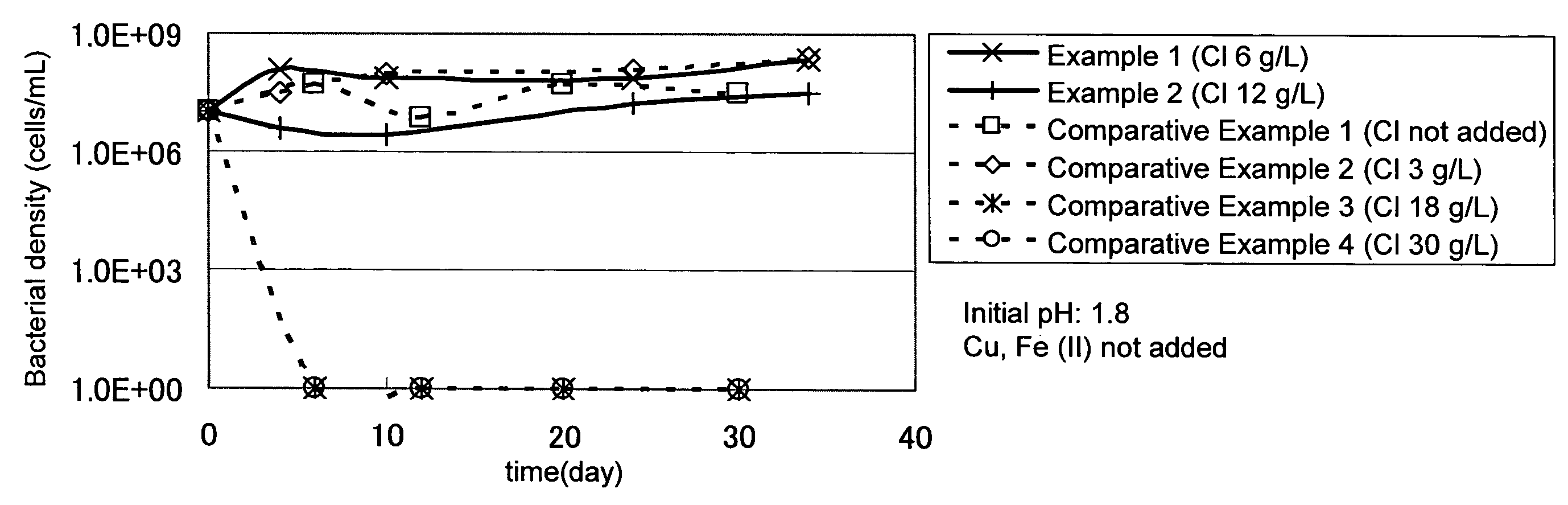
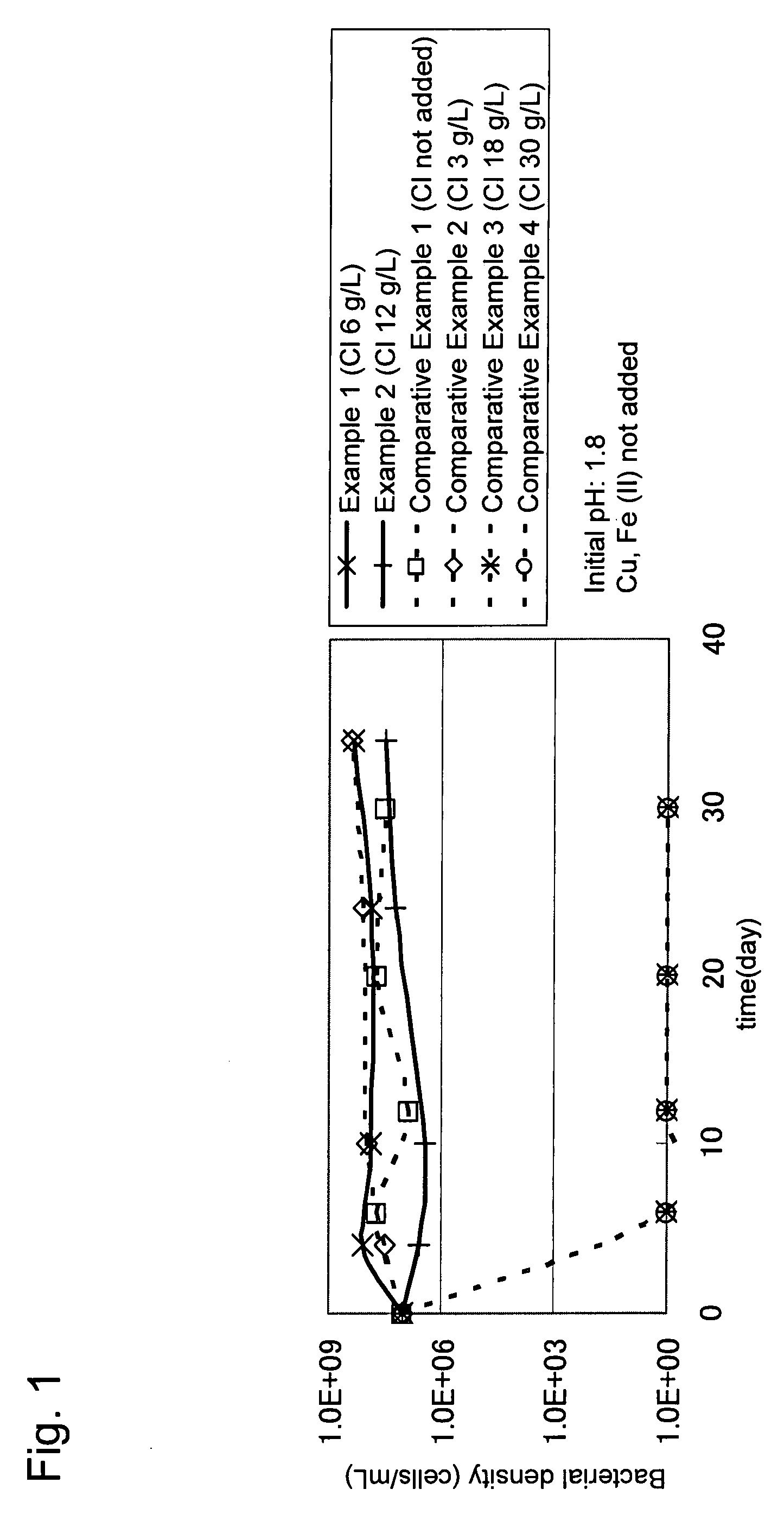
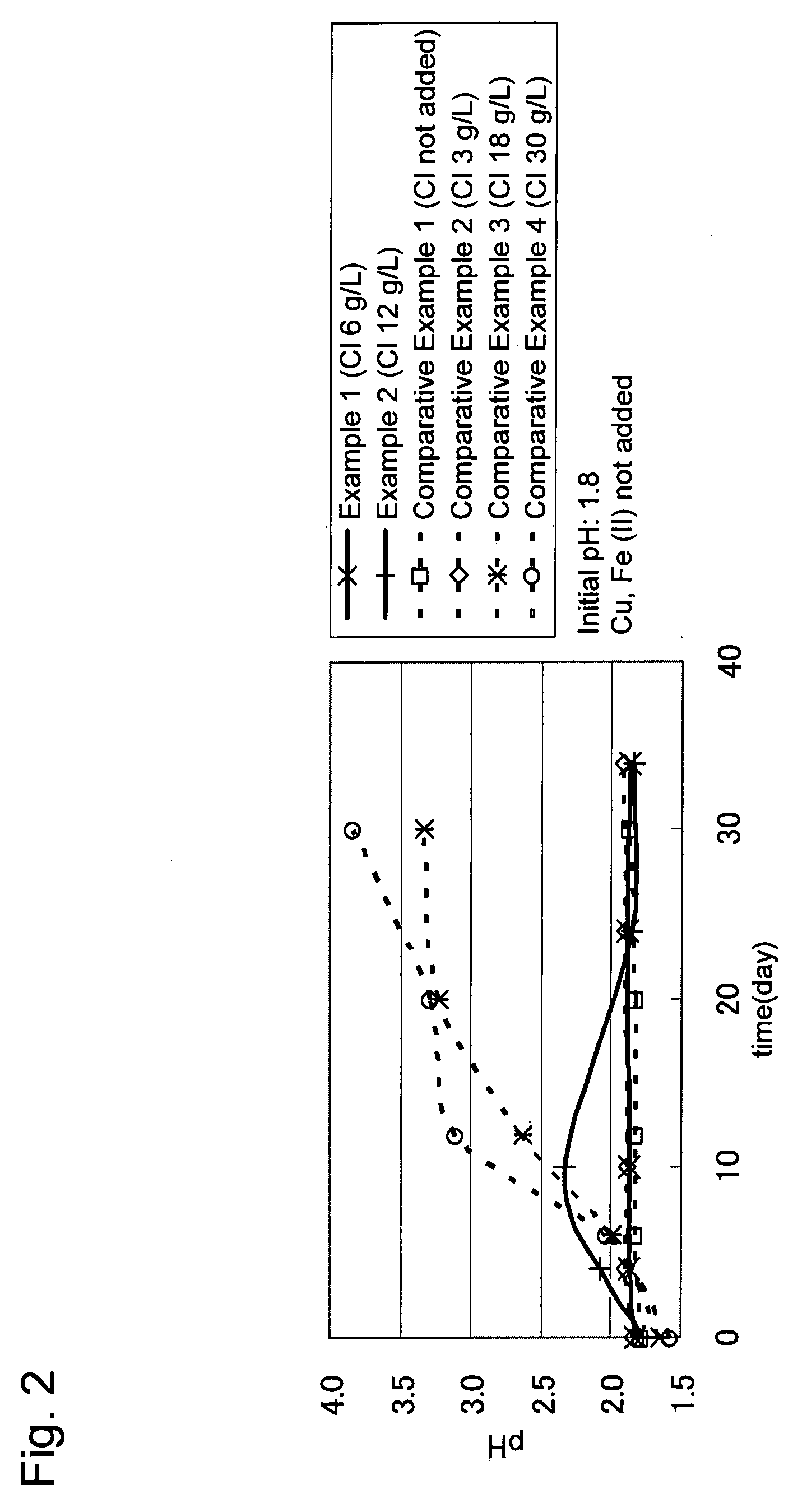
[0034]Concentrate (mined in Candelaria) containing chalcopyrite as a main constituent was used as a target ore. The quality of the concentrate was as follows: Cu=28% by mass; Fe=28% by mass; and S=32% by mass.
[0035]Three grams of the above concentrate was mixed with 300 mL of a leaching solution (containing: ammonium sulfate=3 g / L; potassium hydrogen phosphate=0.5 g / L; magnesium sulfate heptahydrate=0.5 g / L; and potassium chloride=0.1 g / L) that had been adjusted to a pH of 1.8 with sulfuric acid and poured into a 500 mL Shaking flask. To the leaching solution in the flask, sodium chloride was added so that the chloride ion concentration became 6 g / L (Example 1) or 12 g / L (Example 2), and a chloride ion-resistant sulfur-oxidizing bacterium (Acidithiobacillus sp. TTH-19A strain: accession no. NITE P-164) was further added at a density of 1×107 cells / mL. Then the flask was shaken at room temperature for a certain period of time. The bacterial density, pH, and copper concentration of th...
examples 3 and 4
[0043]Shaking leaching was performed at room temperature in the same manner as in Example 1 except that sulfuric acid was added to the leaching solution described in Example 1 so that the leaching solution was adjusted to a pH of 1.6 (Example 3) or a pH of 2.0 (Example 4). The bacterial density and copper concentration of the supernatant of the leaching solution were measured. Then, time course changes in measurement results were examined.
examples 5 and 6
[0048]Shaking leaching was performed at room temperature in the same manner as in Example 1 except that the initial copper (II) ion concentration and the initial iron (II) ion concentration in the leaching solution were adjusted to 0.5 g / L (Example 5) or 1.0 g / L (Example 6). An increase in the copper concentration in the supernatant of the leaching solution was measured. Then, time course changes in the measurement results were examined.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com