Differential labeling for quantitative analysis of complex protein mixtures
a technology of complex protein mixture and quantitative analysis, applied in the direction of assay labels, peptide preparation methods, material testing goods, etc., can solve the problems of difficult to explain the mechanism of biological processes by genomic analysis alone, no protein analytical technology approaches the throughput and level of automation of genomic technology, and achieves high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Peptide Labeling Moiety (or Peptide Encoded Tags, “PEPTags”)
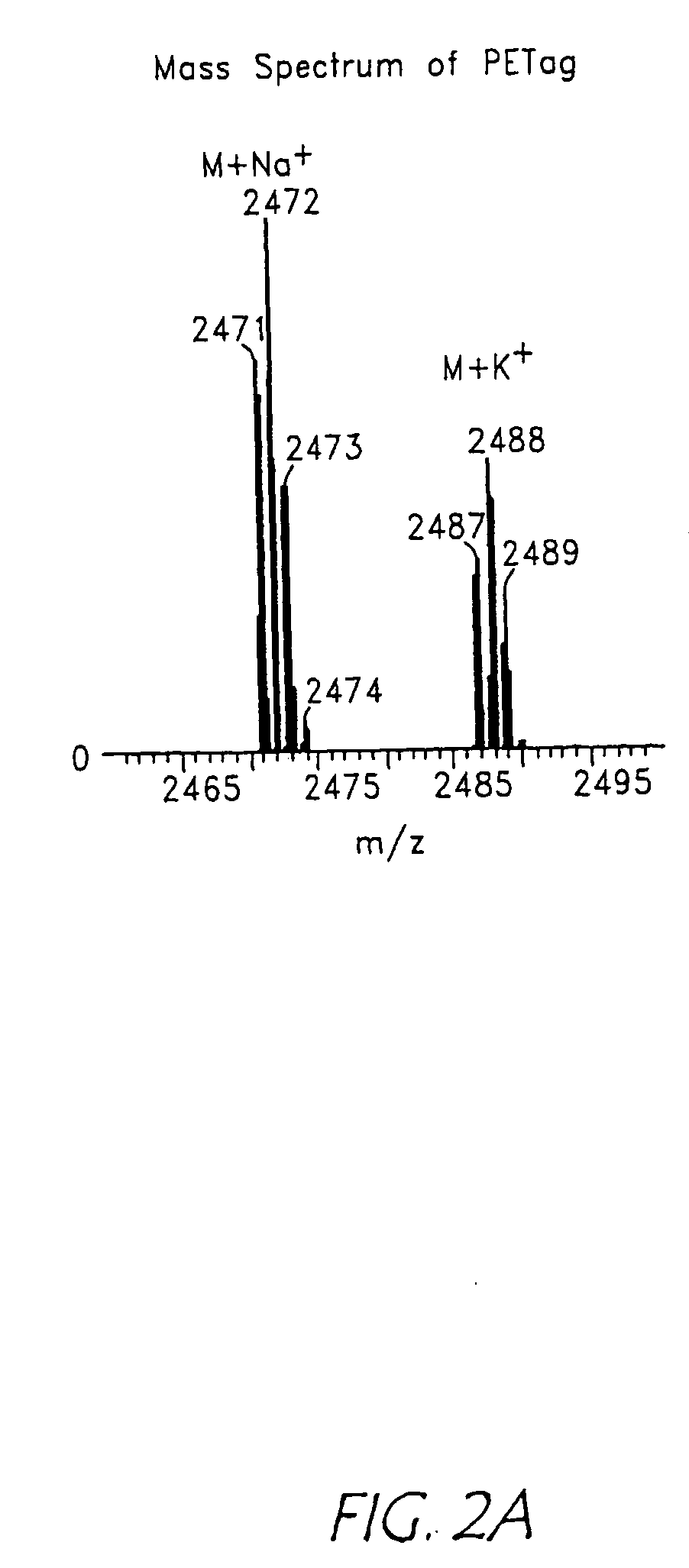
[0421]A pair of PEPTags, described generally above, was synthesized from peptides with following sequences: Ac-AYPYDVPDYASENLYFQGK (SEQ ID NO: 39) and AYPYDVPDYASENLYFQGOrn (SEQ ID NO: 40). In dry DMF containing excess (2-3 molar equivalents) DIEA, each of the peptides was mixed with two molar equivalents of iodoacetic anhydride for 10 min at room temperature under N2 gas, to give Lys-PEPTag and Orn-PEPTag, respectively. The reaction was terminated by adding acetic acid. Solvent was removed by vacuum centrifugation, and the product was purified by reverse-phase FPLC, and analyzed by MAILDI MS (TofEpec 2E, Micromass, Beverly, Mass.) and ESI MS / MS (API 3, PE Sciex, Foster City, Calif.).
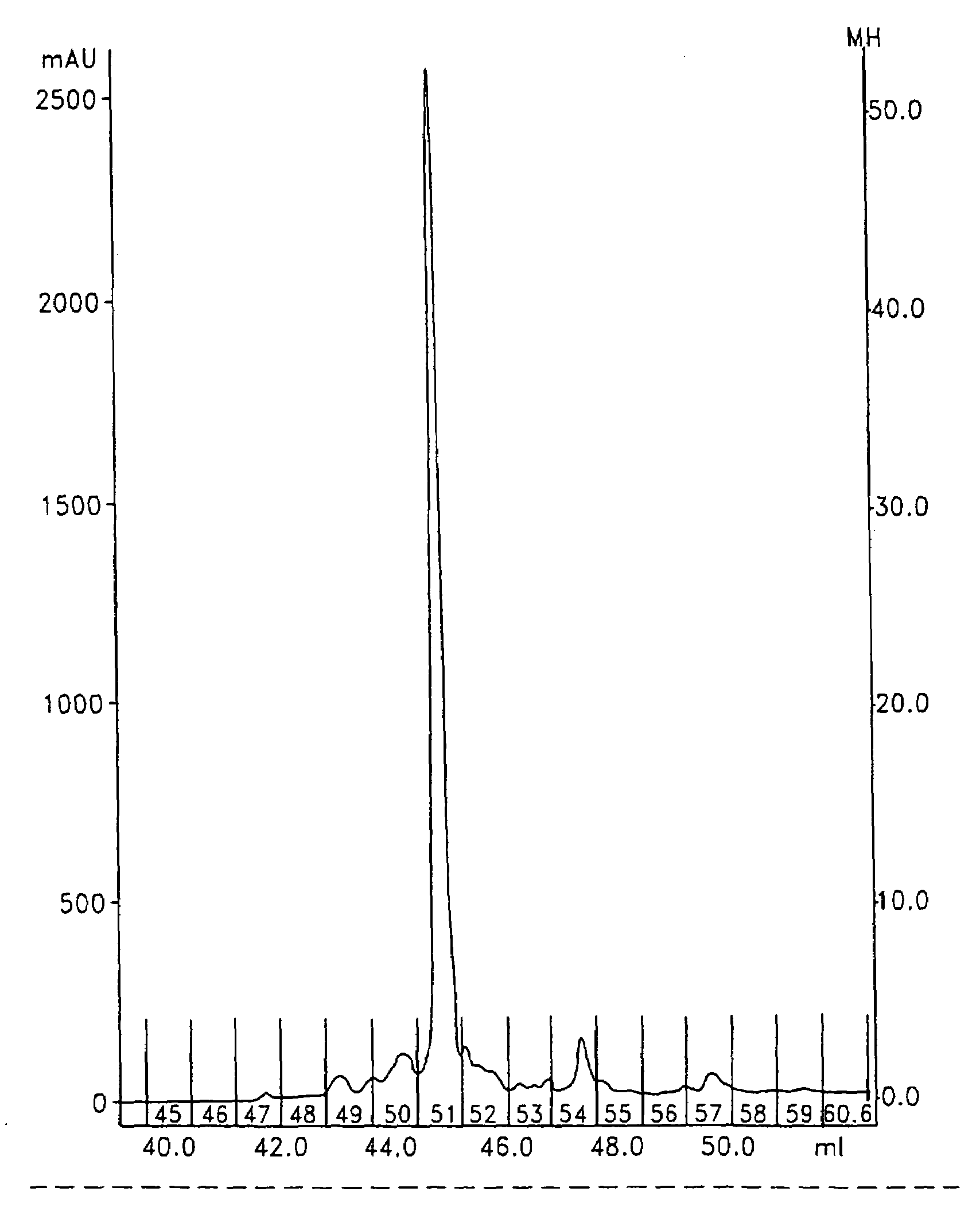
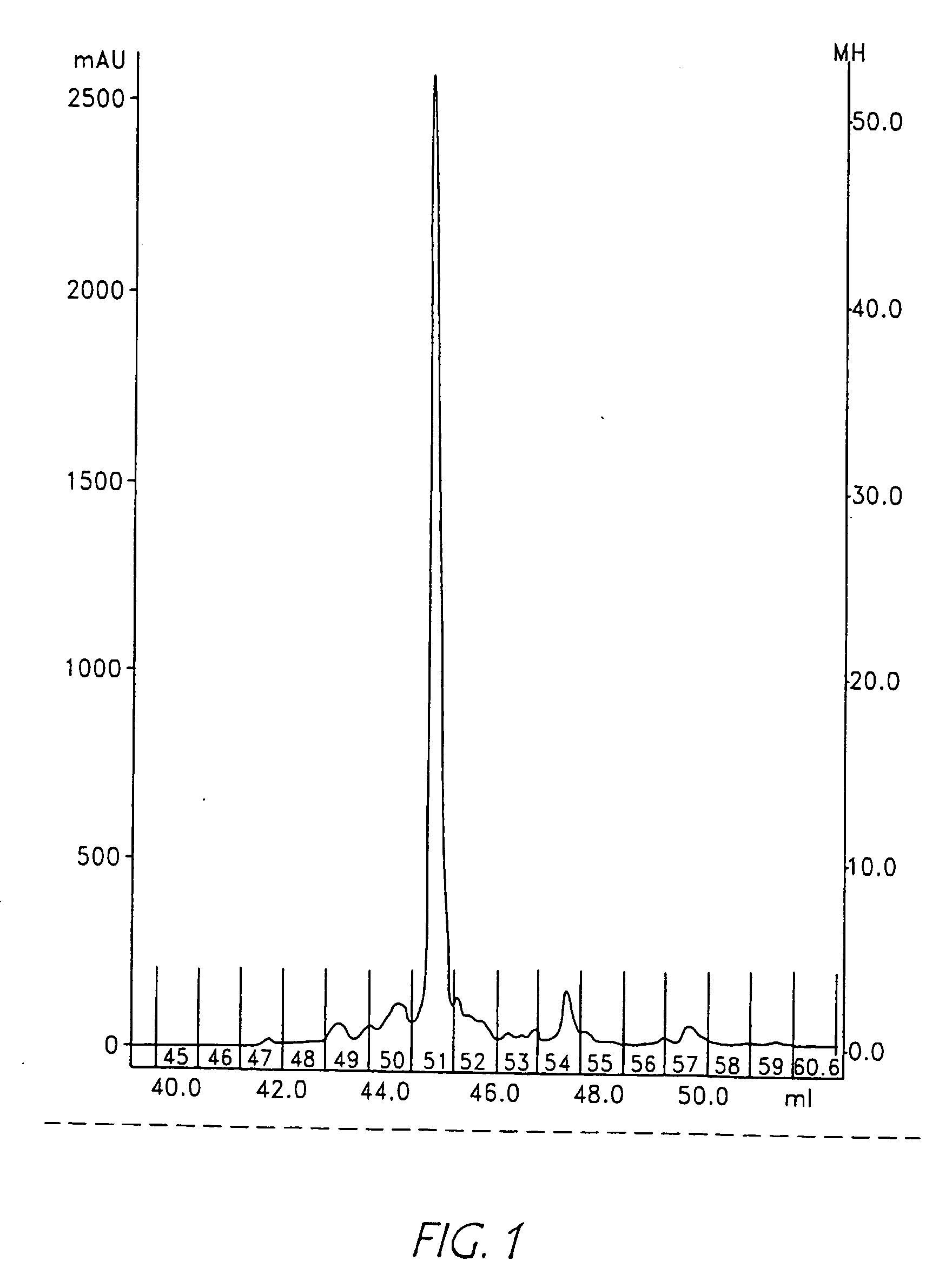
[0422]In order to demonstrate that the mass spectrometric ionization efficiency of the two synthesized peptide tags was essentially equal, the two products were mixed in different ratios and analysed by LC-MS. The ratio of the meas...
example 2
PEPTag Qualitative Protein Analysis
Simplification of Complex Mixtures
[0423]We tested the PEPTag method, described generally herein, on Bovine Serum Albumin (BSA). 200 μL BSA (0.25 mg / mL) was denatured and reduced in a solution containing 0.1% SDS, 5 mM tributyl phosphine and 50 mM Tris buffer (pH 8.5) for 3 min at 100° C. and for 1 hour at 37° C. The side chains of cysteinyl residues were derivatized with a tenfold molar excess of Lys-PEPTag. Tagged protein was digested by trypsin overnight at 37° C. Trypsin activity was quenched with trypsin inhibitor and the peptide mixture bound to anti-HA affinity matrix for 2 hours at 4° C. The anti-HA resin with bound peptides was washed in equilibration-buffer (20 mM Tris, pH 7.5; 0.1 M NaCl; 0.1 mM EDTA), 3×10 min. at 4° C. The bound peptides were cleaved from the matrix by incubation with TEV protease for 6 hours at 30° C. The cleaved peptides were analyzed by either Matrix Assisted Laser Desorption Ionization Mass Spectrometry (MALDI MS), ...
example 3
PEPTag Quantitative Protein Analysis
Differential Labeling
[0426]We tested the PEPTag quantitative strategy on two mixtures containing the same two proteins at different concentrations. Mixture 1 had 500 pmol BSA (0.1 mg / mL) and 400 pmol β-lactoglobulin (0.1 mg / mL) and was reacted with 9 mmol Lys-PEPTag. Mixture 2 had 250 pmol BSA (0.05 mg / mL) and 800 pmol β-lactoglobulin (0.2 mg / mL) and was reacted with 9 nmol Orn-PEPTag. Protein denaturation, reduction, tagging, and digestion were the same as described above. The two samples were combined after tryptic digestions, and bound to anti-HA matrix. TEV digestion and MS analysis were as described in Example 2. Peptides were quantified by measuring, in the MS mode, the relative signal intensities for pairs of peptide ions of identical sequence, tagged with Lys or Orn-PEPTags, respectively. The results are shown in FIGS. 6, 7, and 8 and the following table.
Peptide sequenceObservedExpectedProteinidentifiedratioMean ± S.D.ratioBovineSLHTLFGDEL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| Ph | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com