Topical product formulations containing strontium for reducing skin irritation
a technology of strontium and product formulation, which is applied in the direction of biocide, drug composition, hair cosmetics, etc., can solve the problems of reducing the effect of skin irritation, affecting the effectiveness of topical products, and affecting the effectiveness of skin irritation, so as to suppress skin irritation, prevent, reduce or eliminate the effect of potential irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Clinical Studies of Anti-Irritation Activity
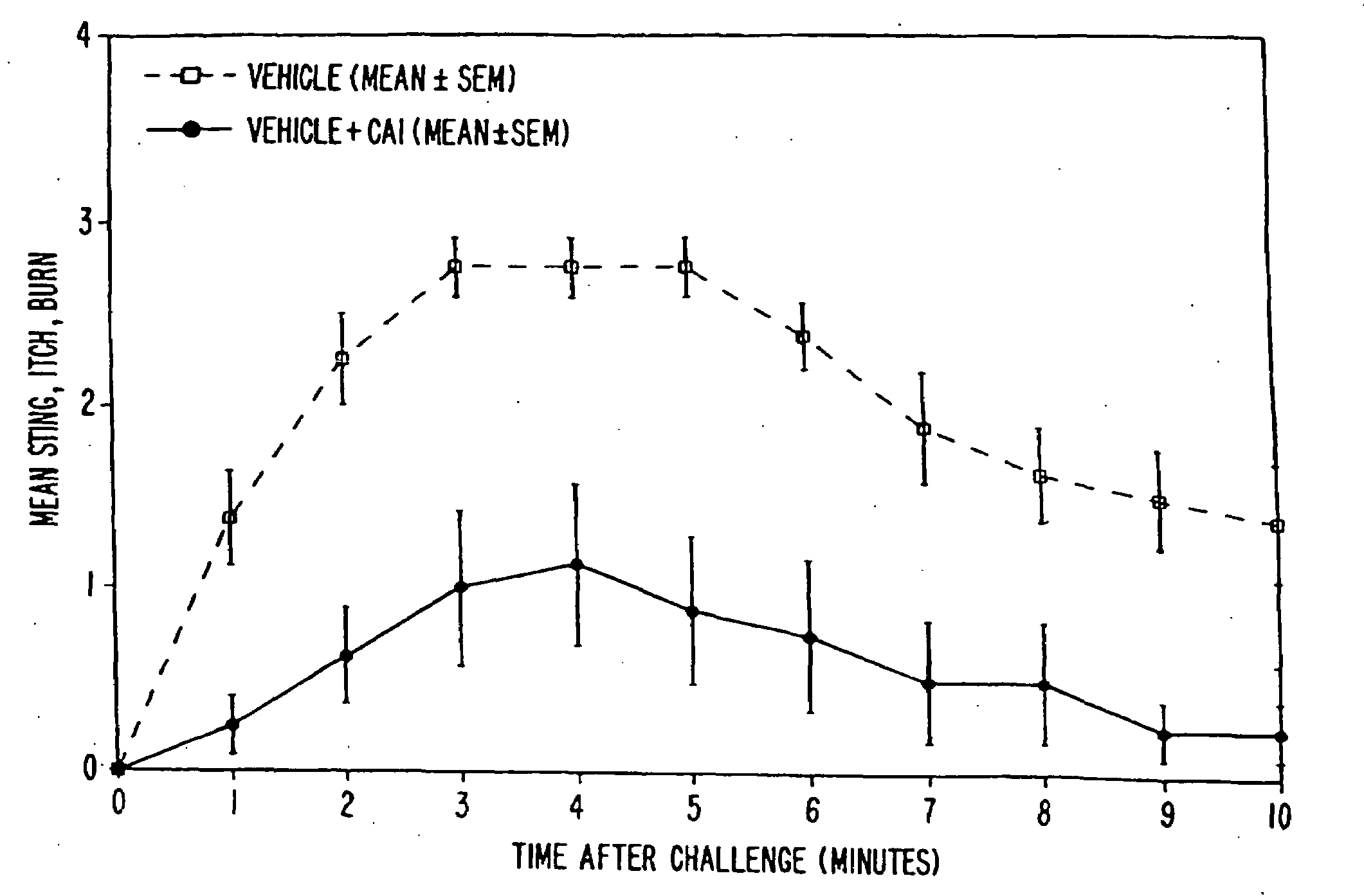
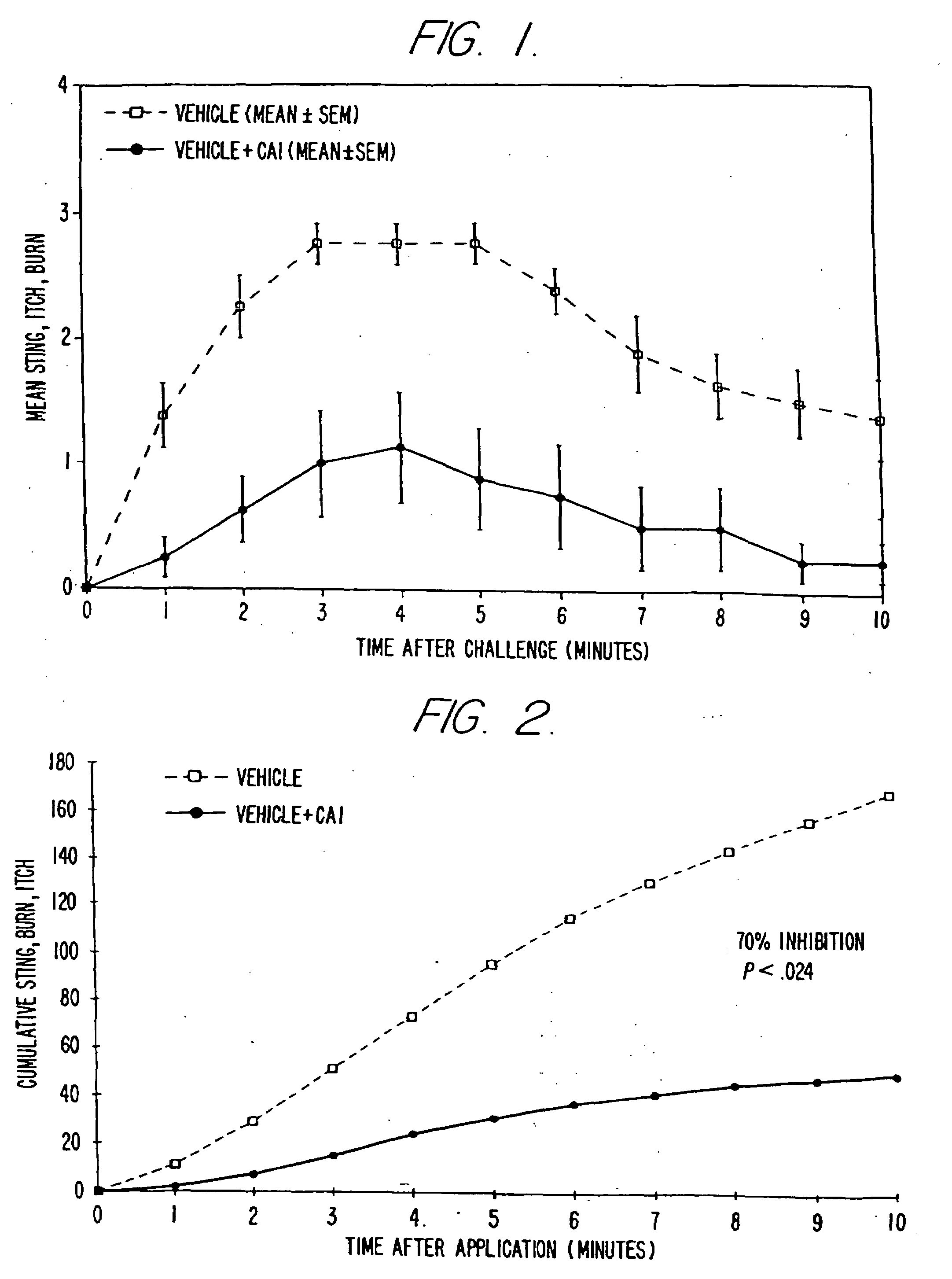
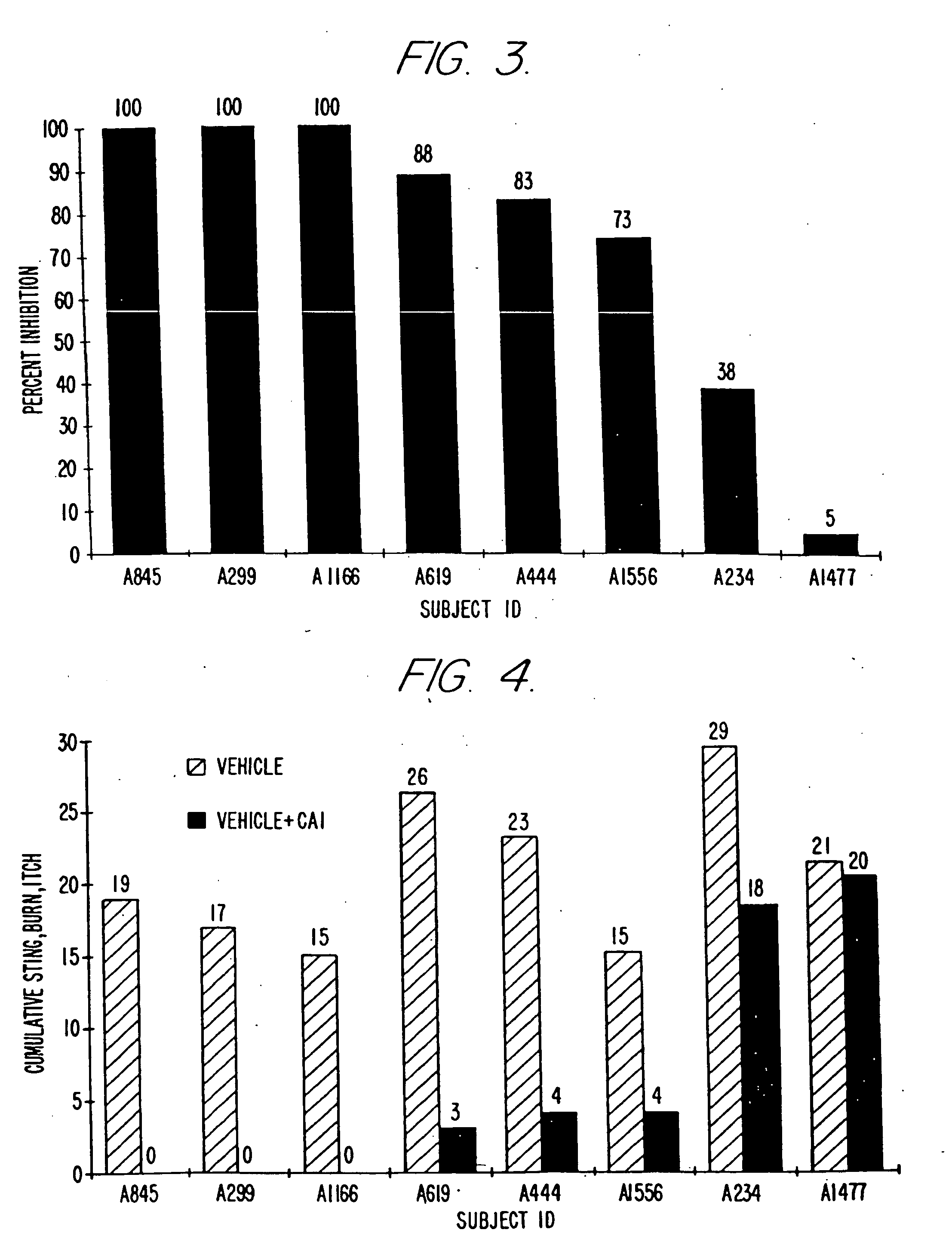
[0091]The objective of the clinical trials was to determine whether and to what extent topical formulations of the strontium cation reduced or prevented skin irritation caused by certain severe skin irritants, including particularly lactic acid and glycolic acid (which are hydroxy acids), capryloyl salicylic acid (a β-hydroxy acid ester) and capsaicin (an isolate from cayenne and paprika known for its skin-irritating properties). The trials were conducted in a double blind, randomized, vehicle-controlled manner. Various formulations of the invention were tested in over 740 people. The results confirm the highly reproducible anti-irritant activity of the formulations of the present invention.
[0092]a. Lactic Acid Irritation Trials
[0093]1. Protocol
[0094]The majority of the trials were conducted using lactic acid as the skin irritant, and proceeded generally as follows.
[0095]The subjects were women who had been screened and shown to exhibit no...
example 2
Dose-Response Studies
[0129]Additional studies of anti-irritant activity using varying concentrations of strontium cations were conducted in order to assess the dose-response behavior of the present formulations. The lactic acid irritation protocol described above was used in which the anti-irritant cation component was strontium nitrate (31-500 mM). Cumulative irritation inhibition data are set forth in the following table, and are depicted graphically in FIG. 25.
Concentration (mM)Percent Inhibition31276232125422507250082
example 3
Additional Formulation Examples
[0130]Cation salts of the invention were formulated at various concentrations in a number of commercially available topical vehicles, and also in various commercially available topical cosmetic products. The resulting mixtures generally did not alter the texture, color, consistency or other physical properties of the product, and could be used as formulations to inhibit topical irritation.
[0131]a. Silicone-Based Vehicles
[0132]A 500 mM strontium nitrate topical lotion was prepared as follows. 10.58 g of strontium nitrate was dissolved in 55 ml of deionized water. This solution was combined with 10 ml cyclomethicone (Dow Corning, “DC344”), 20 ml cyclomethicone / dimethiconol (Dow Corning, “DC1401”) and 15 ml cyclomethicone / dimethicone copolyol (Dow Corning, “DC3225C”) and blended for 2-3 minutes. Imidizolidinyl urea (0.5%) was added as a preservative. An opaque white lotion (100 ml) resulted which, when applied to the skin of a fair (olive) skinned individ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com