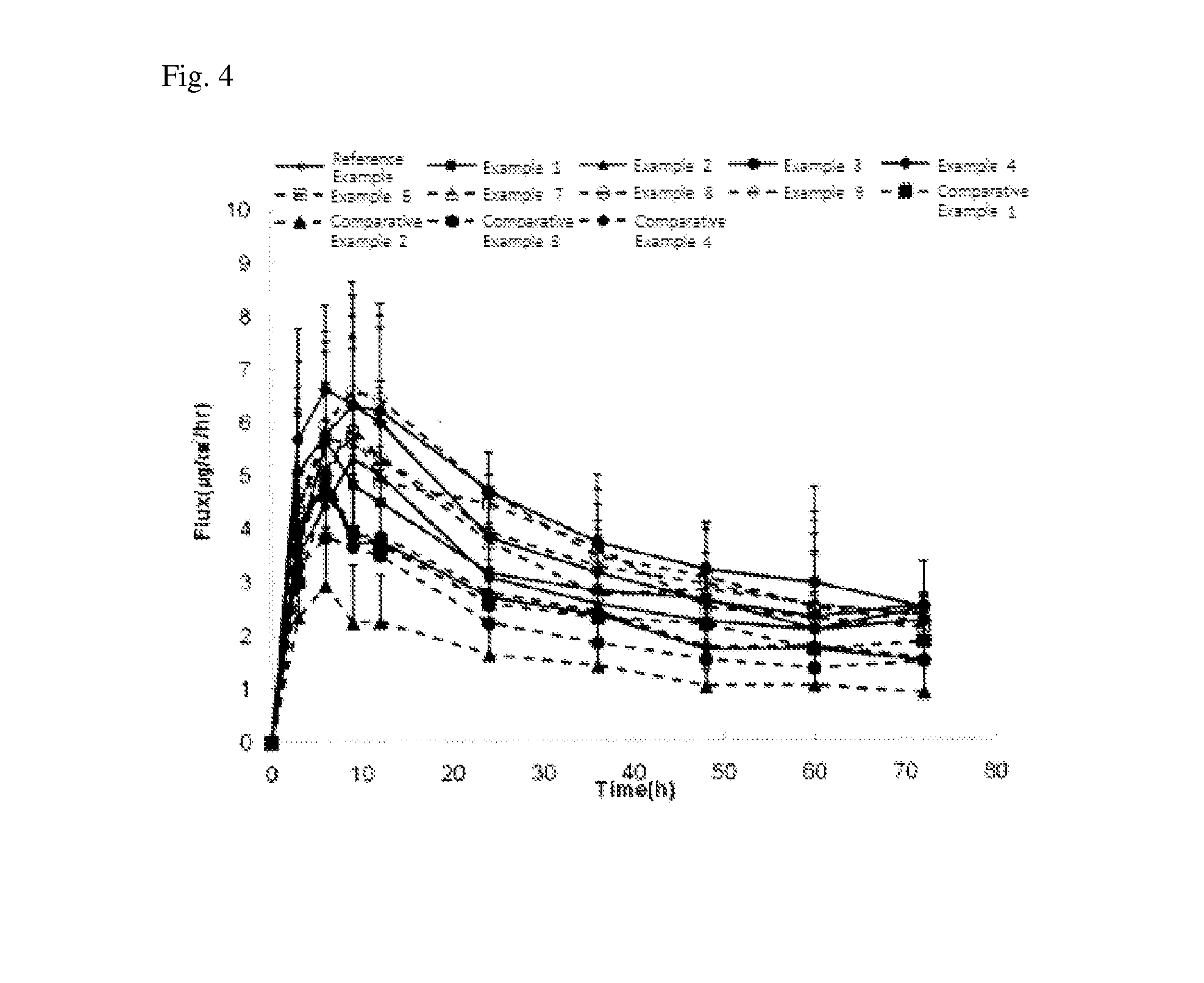
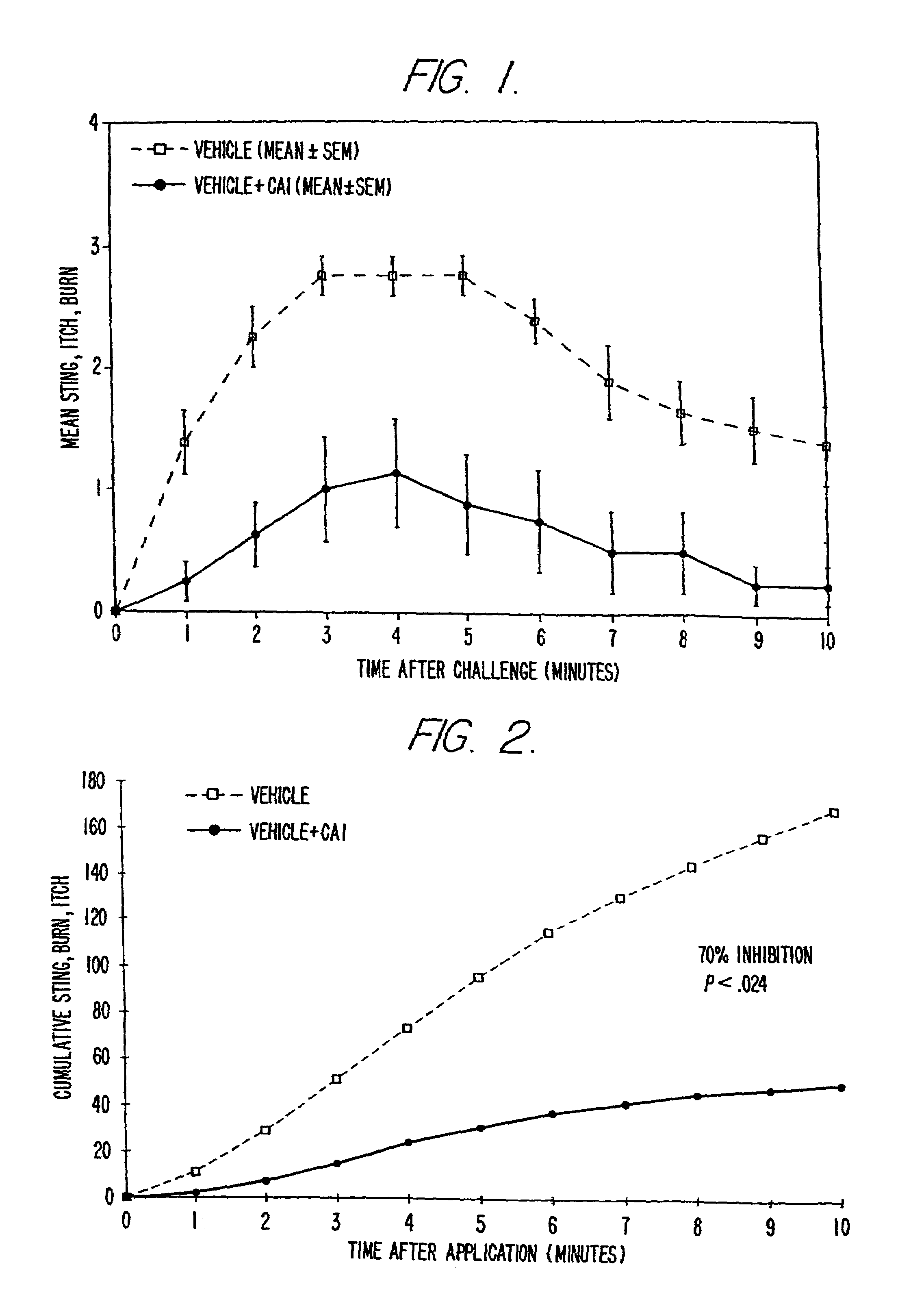
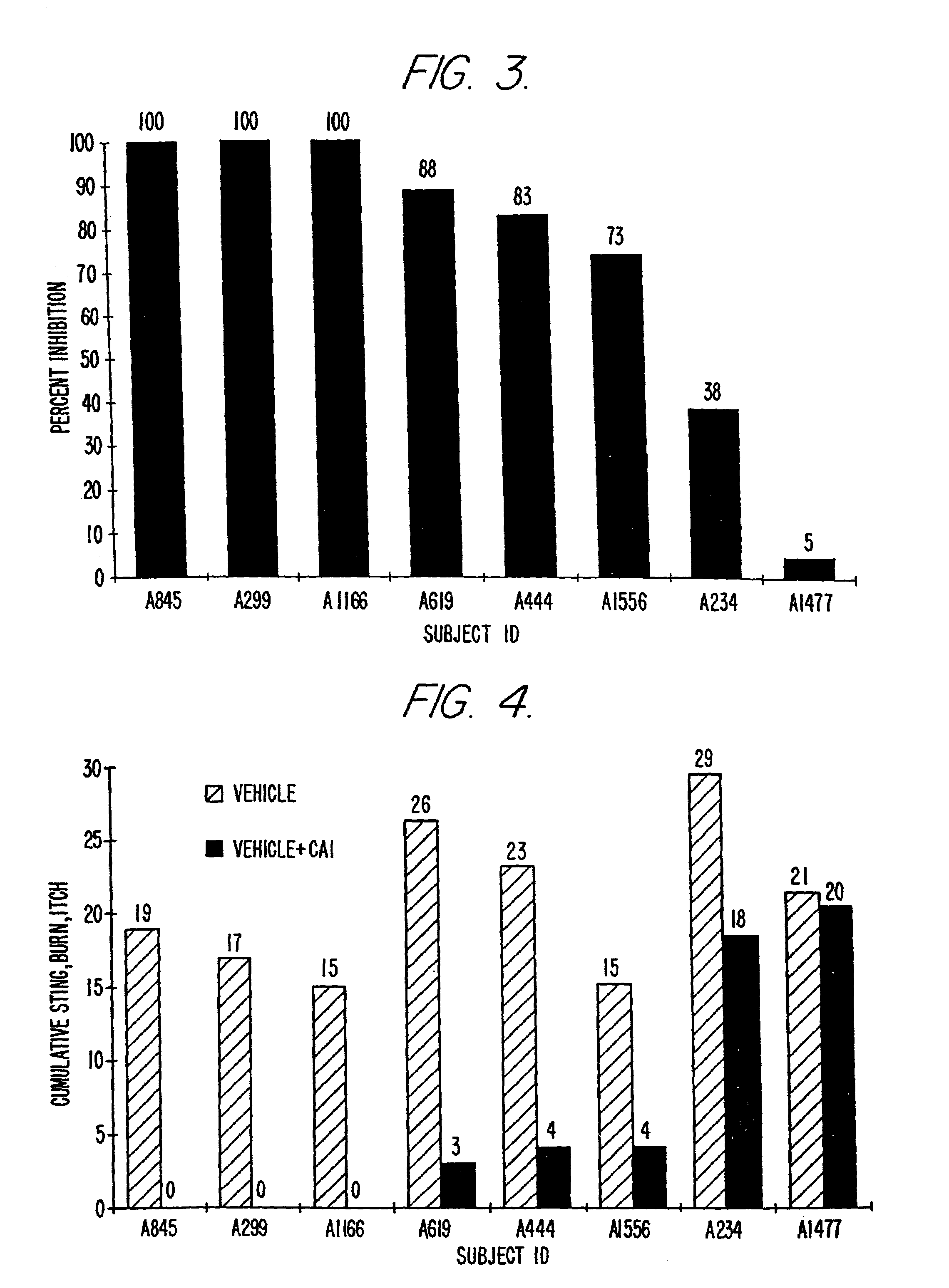
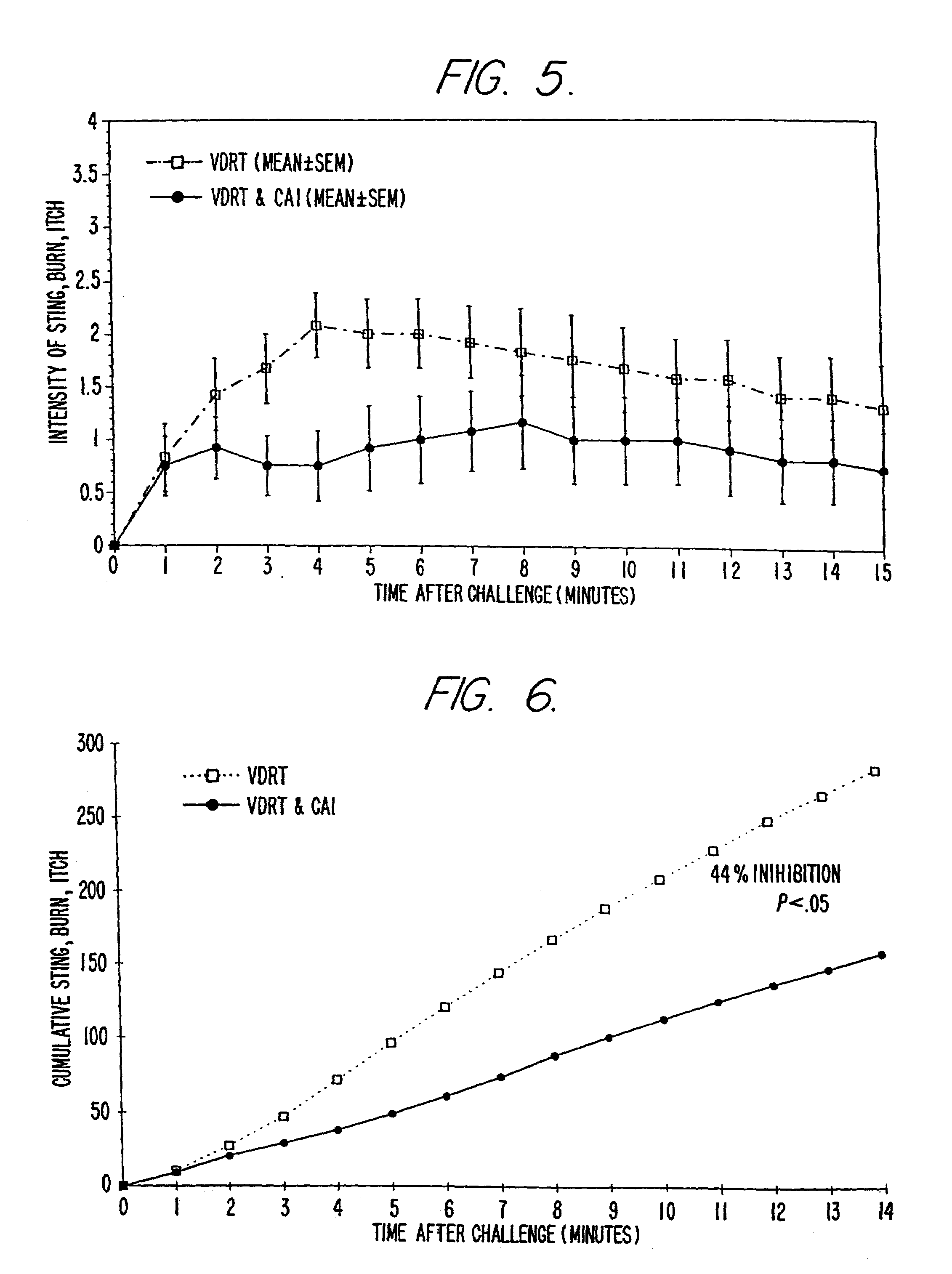
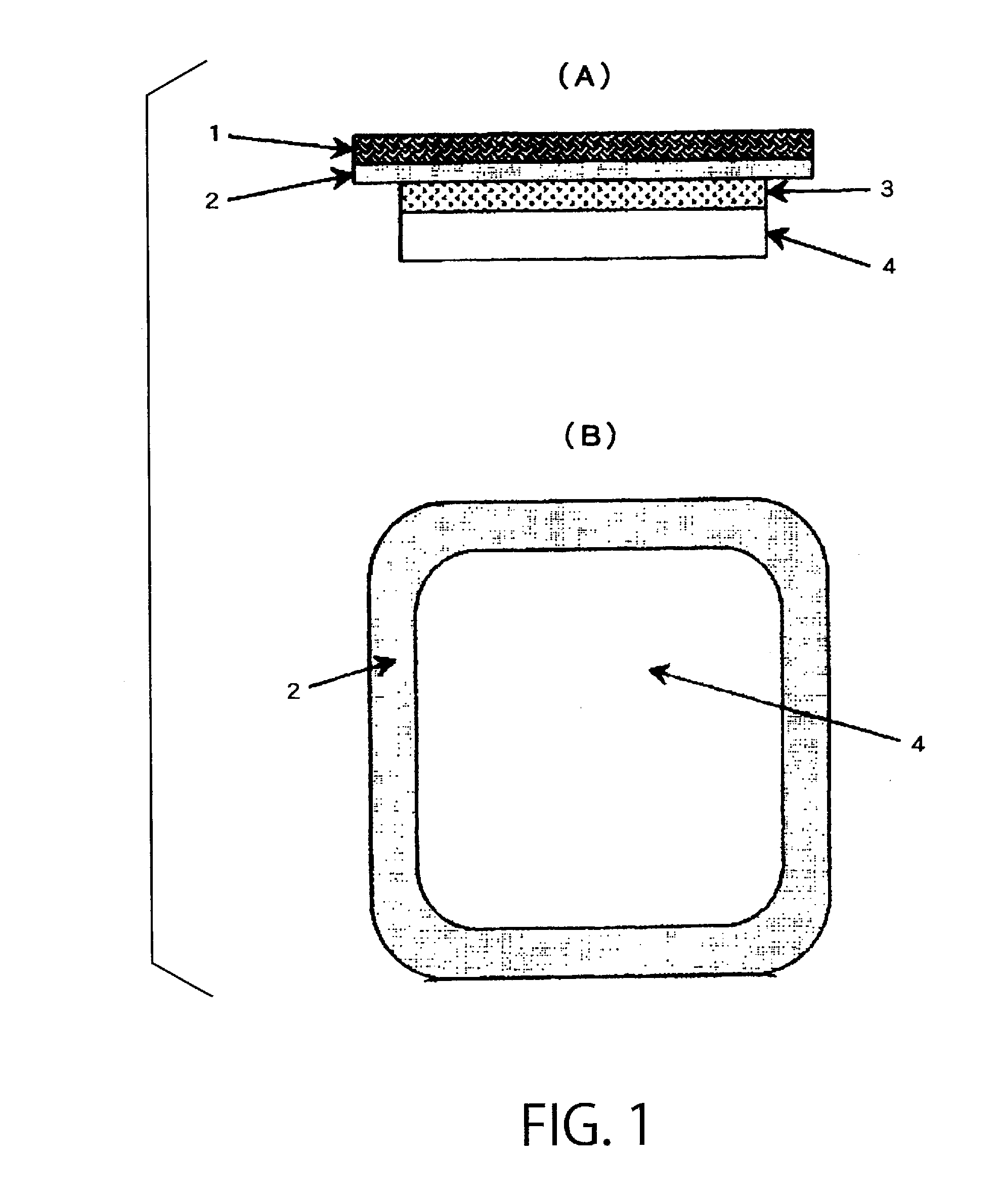
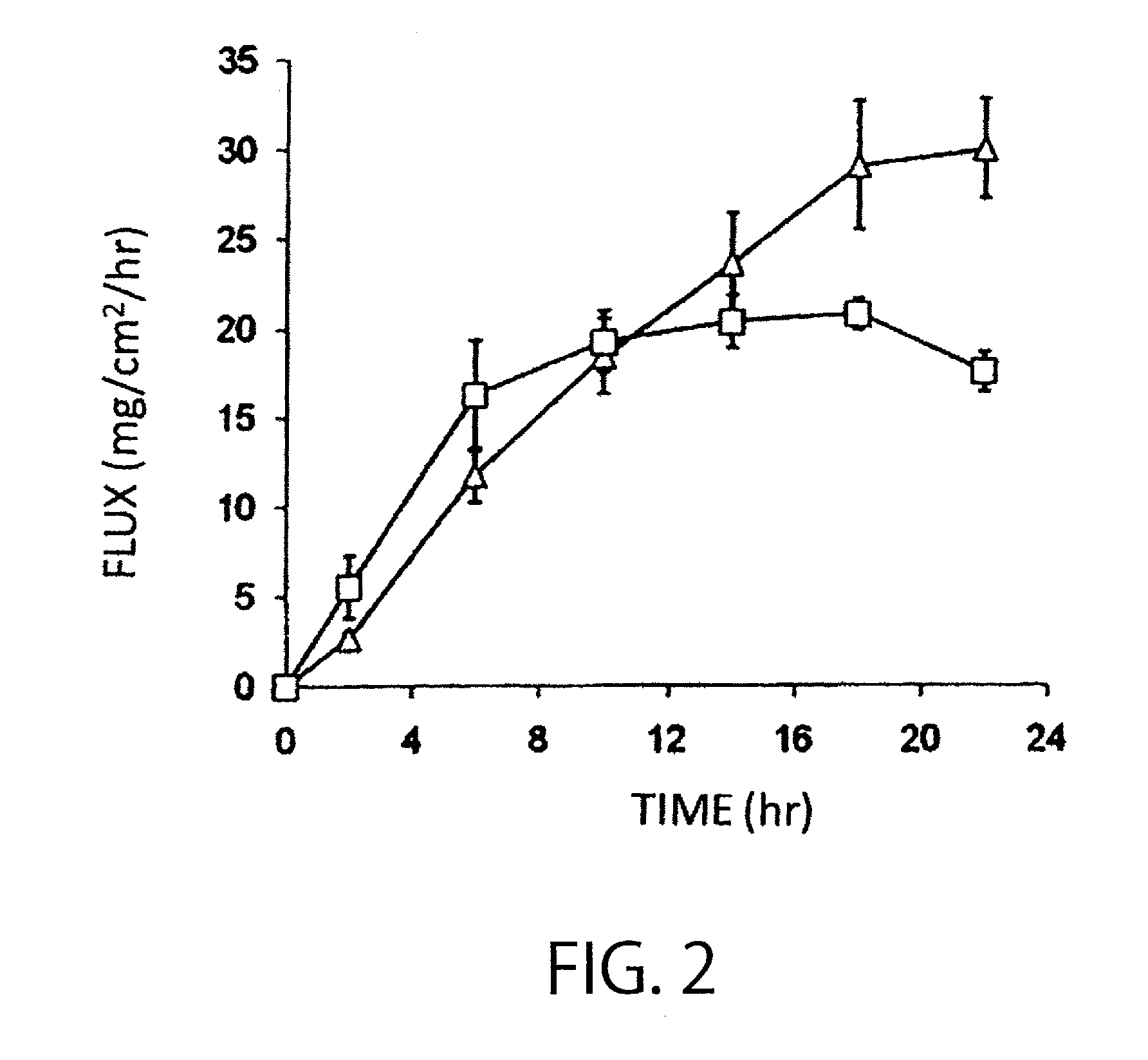
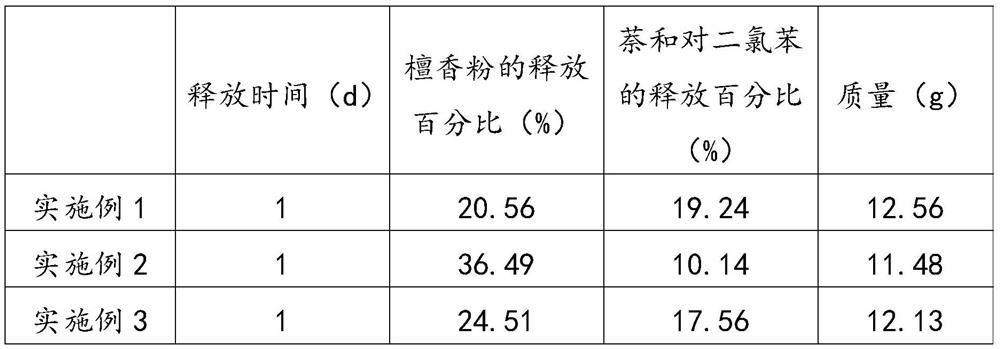
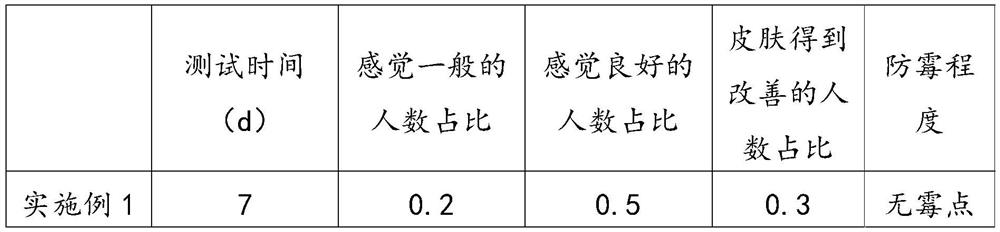
Patents
Literature
40results about How to "Suppress irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
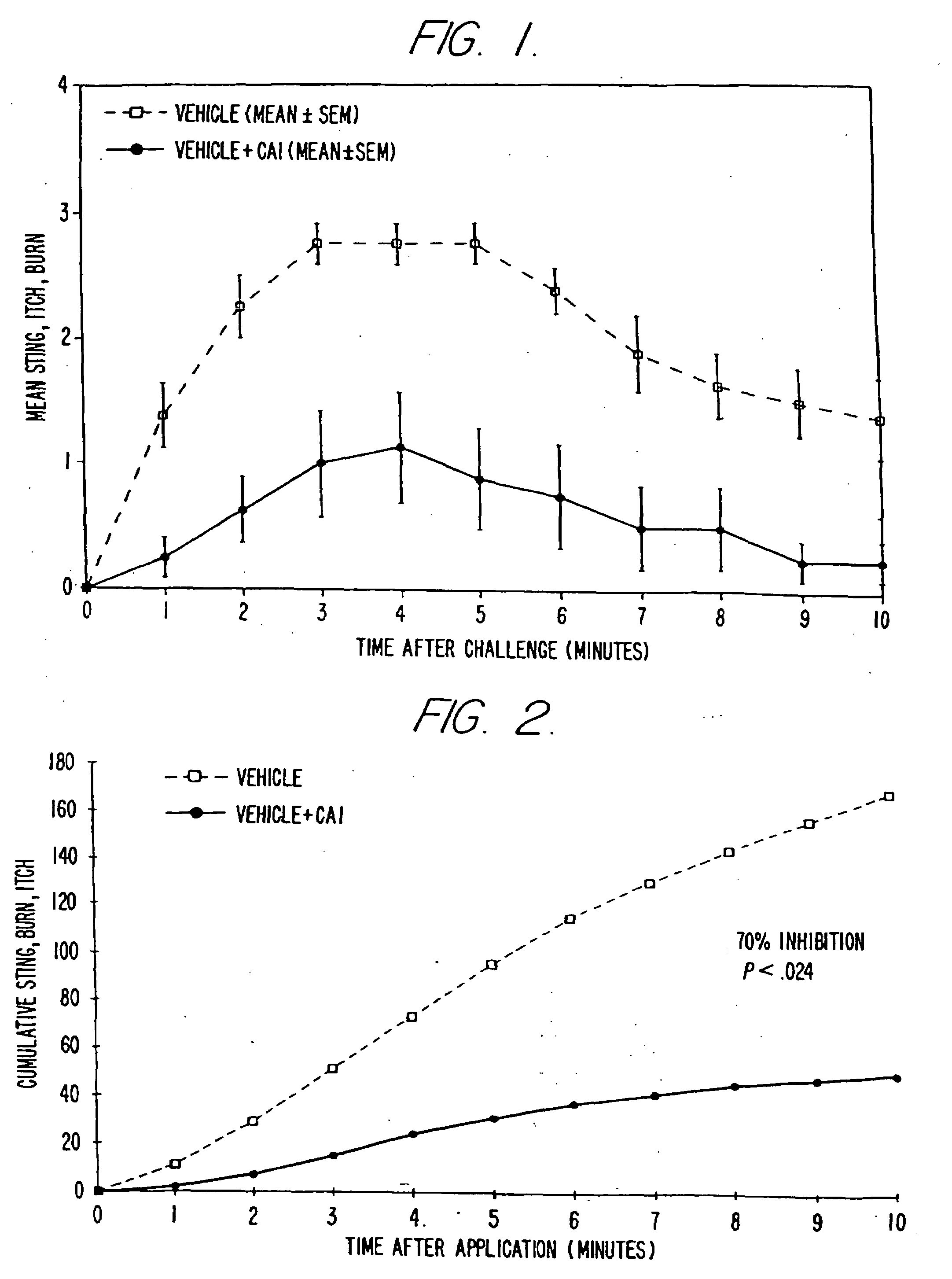
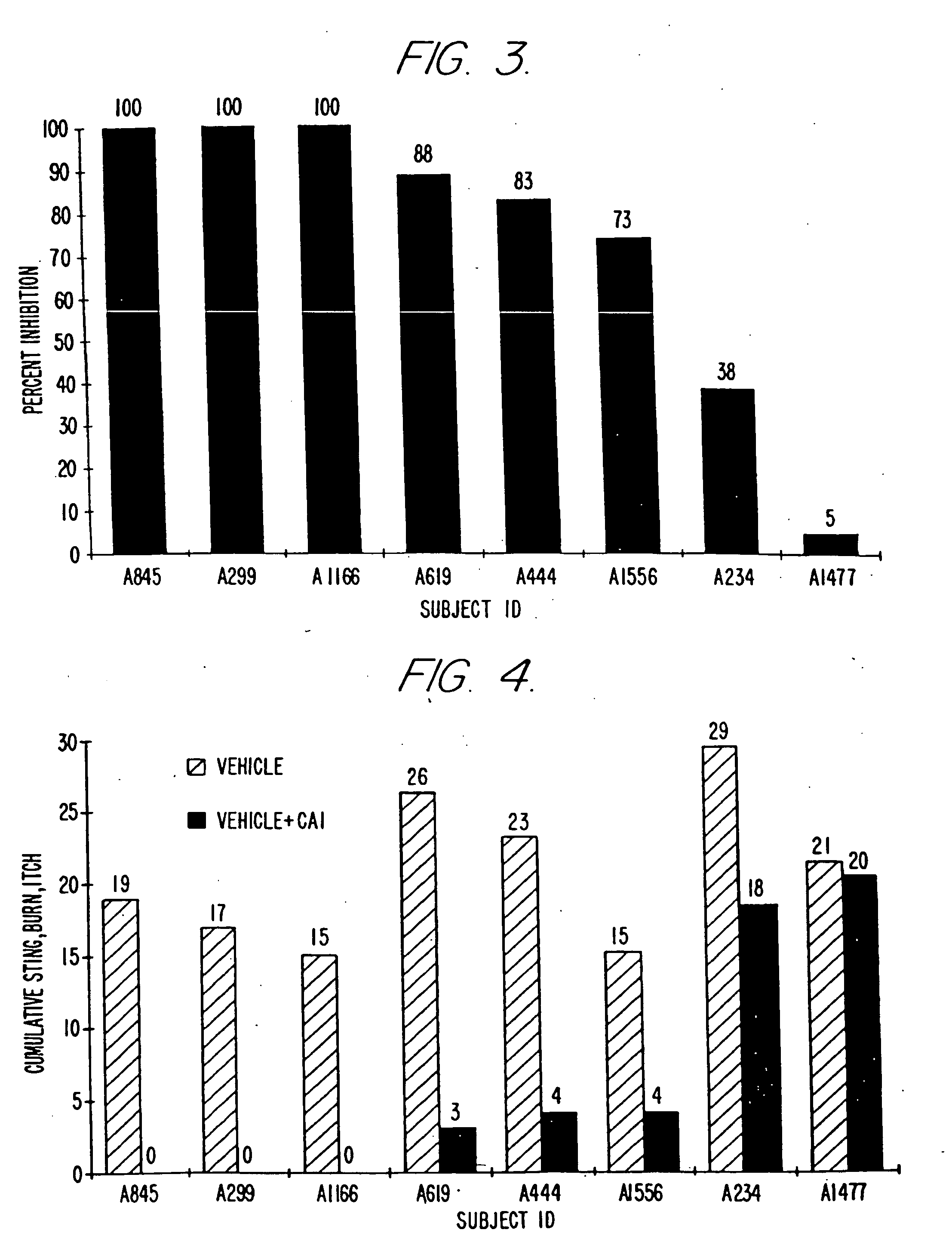
Antimicrobial and antiseptic methods using antimicrobial composition
InactiveUS6159999ASuppressing skin irritationImprove stabilityBiocideHydroxy compound active ingredientsSolventSkin irritant
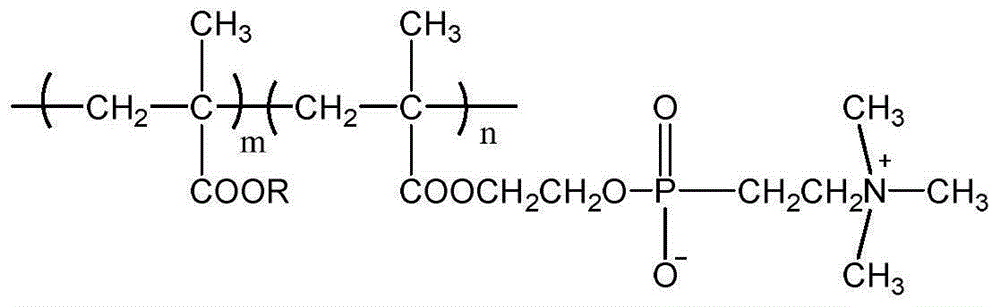
An antimicrobial composition is provided in which the skin irritation of isothiazolone type compound is decreased and the injection operation properties thereof is improved by forming it as an uniform solution. The composition contains isothiazolone type compound and a compound which decreases the skin irritation of the former in a molar ratio of 1:0.1-50 and is dissolved in a solvent.
Owner:KURITA WATER INDUSTRIES LTD
Contact lens distribution/storage method and contact lens package
InactiveUS20120006695A1Suppress fluctuationsLarge buffering capabilityOther chemical processesOther accessoriesContact lensPhysics
A contact lens distribution / storage method with which contact lenses can be reliably stored in a small space. To achieve this with a method for storing contact lenses for distribution using a contact lens package containing a packaging solution and the contact lens, a soft contact lens is used as the contact lens. A fluid volume of the packaging solution is 0.1 to 1.0 mL, and a buffering capability of the packaging solution is 3 mmol / L or greater measured in buffering capacity.
Owner:MENICON CO LTD
Immunoregulatory structures from normally occurring proteins
ActiveUS20110262470A1High proliferation rateInhibitory activityCompound screeningApoptosis detectionADAMTS ProteinsBinding peptide
The present invention relates to isolated protein sequences that correspond to cell binding peptides, fragments, neo-structures and / or neo-epitopes of a normally occurring serum protein present in human tissue, wherein the peptide, fragment, neo-structure and / or neo-epitope has an immunoregulatory activity and is the result of either an enhanced proteolytic activity and / or conformational changes in a tissue, or a malignant tumour. In the present patent application, a common structure of several of these peptides, fragments, neo-structures and / or neo-epitopes, having immunoregulatory activity by binding to receptors on immune cells, has been identified. The present invention further also relates to monoclonal and / or polyclonal antibodies directed to a cell binding fragment of a normally occurring serum protein present in human tissue, as described above.
Owner:CANIMGUIDE THERAPEUTICS
Fentanyl transdermal patch
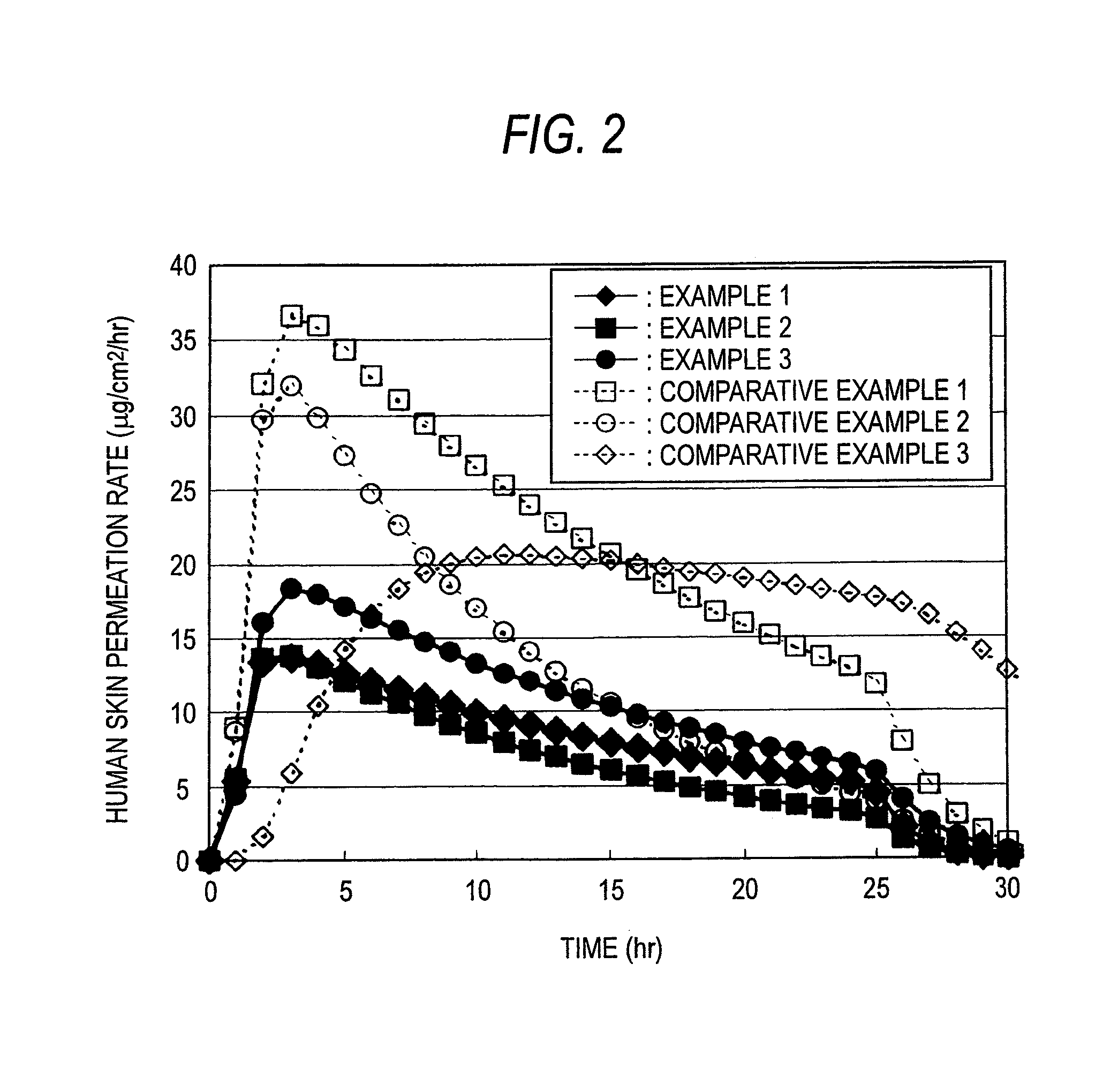
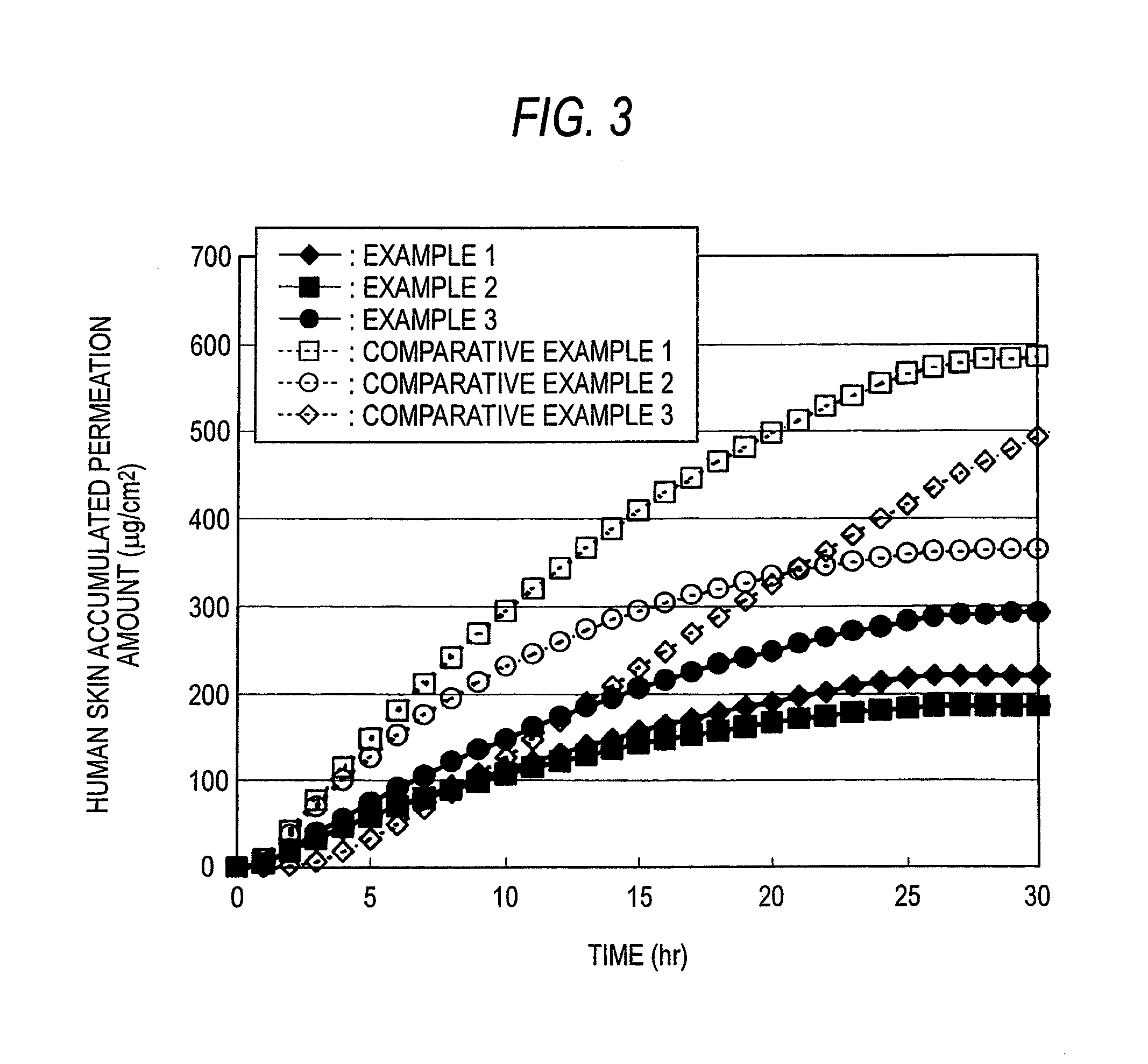
ActiveUS20140005617A1Maintain constant fentanyl skin permeabilityPrevent disengagementBiocideAdhesive dressingsSkin permeabilityAcrylic rubber
Provided is a fentanyl transdermal patch comprising an acrylic-rubber hybrid as a drug-adhesive layer. The fentanyl transdermal patch can maintain constant fentanyl skin permeability for three days by maintaining close contact with the skin such that desorption, release by moisture and sweat, and skin stimulation are all improved.
Owner:ICURE
Topical product formulations containing strontium for reducing skin irritation
InactiveUS7404967B2Inhibit and suppress skin irritating propertyFast-acting, efficient and safe topical skin anti-irritant effectsHeavy metal active ingredientsCosmetic preparationsWater solubleStrontium Cation
Owner:COSMEDERM BIOSCI +1
Non-irritating compositions containing zinc salts
InactiveCN101163455AReduce sensitivityInhibit stimulusPowder deliveryCosmetic preparationsIrritationOrganic chemistry
The present invention relates to methods and compositions which employ low concentrations of combinations of zinc salts to prevent the irritation of skin or mucous membranes that may be caused by therapeutic agents, by personal hygiene products, by articles such as gloves or condoms, or by various physical, chemical, mechanical, or biological irritants.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Skin External Preparations
To provide skin external preparations characterized in that the initial permeation rate of oil-soluble drugs (e.g. retinol and tocopherol acetate) is controlled to achieve their sustained release to thereby reduce skin irritation and that they also possess a fresh feel while featuring high degrees of safety and stability. A skin external preparation which comprises a finely dispersed, oil-soluble drug containing wax composition, the composition containing a solid or semisolid wax, a nonionic surfactant, an aqueous dispersion medium, and an oil-soluble drug, the mass ratio of the nonionic surfactant to the wax being 1.0 or more, and the wax, with the oil-soluble drug contained therein, being finely dispersed in solid or semisolid form in the aqueous dispersion medium.
Owner:SHISEIDO CO LTD
Topical product formulations containing strontium for reducing skin irritation
InactiveUS20080131386A1Fast-acting and efficient and safeSuppress skin irritationBiocideHeavy metal active ingredientsWater solubleStrontium Cation
Topical formulations containing aqueous-soluble divalent strontium cation in a suitable topical formulation vehicle, and methods of using these formulations to inhibit skin irritation, are disclosed.
Owner:COSMEDERM TECH
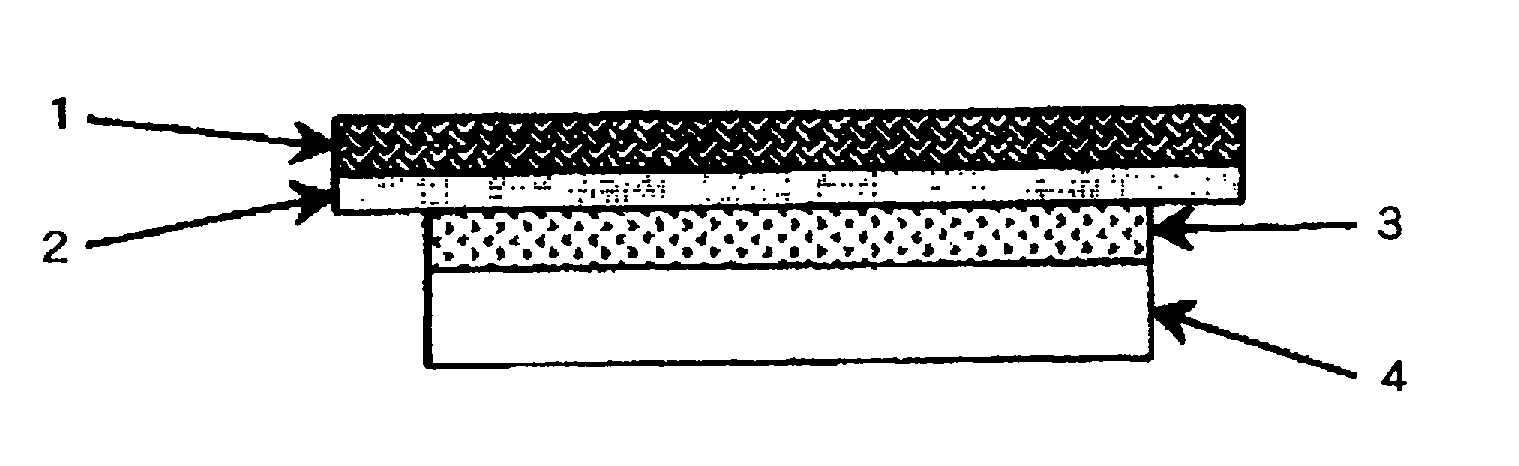
Medical adhesive and medical adhesive tape or sheet
ActiveUS20070275239A1Appropriate adhesivenessEasy to fixAdhesive dressingsAbsorbent padsPolymer scienceMeth-
The present invention provides a medical adhesive and a medical adhesive tape or sheet superior in the fixing performance, particularly perspiration-resistant fixing, which shows low skin irritation and suitable adhesive strength. The present invention provides a medical adhesive containing a water-dispersed copolymer obtained by copolymerizing 100 parts by weight of a monomer mixture containing (meth)acrylic acid alkyl ester and 0.005-2 parts by weight of a silane monomer copolymerizable with the ester, and an organic liquid component compatible with the copolymer; a medical adhesive containing a water-dispersed copolymer obtained by copolymerizing a monomer mixture containing (meth)acrylic acid alkyl ester and a silane monomer copolymerizable with the ester, and an organic liquid component compatible with the copolymer, wherein the gel fraction after crosslinking of the copolymer is 40-80 wt %, and the weight average molecular weight of a sol component after crosslinking is not less than 300000; and a medical adhesive tape or sheet having the medical adhesive at least on one surface of a support.
Owner:NITTO DENKO CORP
Percutaneous absorption preparation comprising Anti-dementia drug
InactiveUS20130337021A1Efficient transdermal administrationReduce skin irritationBiocideNervous disorderIrritationCarboxylic salt
Owner:GOTO TAKESHI
Food therapy composition capable of preventing and treating diabetes and making method thereof
ActiveCN102342506AAggravate the conditionSuppress irritationMilk preparationDough treatmentGramDiabetes mellitus
The invention discloses a food therapy composition capable of preventing and treating diabetes and a making method thereof. The food therapy composition is prepared from the following raw materials by weight: 3 to 30 grams of pear peel, 3 to 30 grams of green tofu skin, 3 to 30 grams of chicken's gizzard-membrane, 0.05 to 0.1 gram of cinnamon, 1 to 2 grams of sharp-leaf galangal fruit, and 1 to 2grams of raspberry. The food which has good mouthfeel and is suitable for daily meals of diabetes is prepared by the 'medicinal and edible' material formula.
Owner:李传梅
Percutaneous administration device of bisoprolol
InactiveUS20100098747A1Suppress irritationReduce skin irritationOrganic active ingredientsBiocideBisoprololLiving body
The present invention relates to a percutaneous administration device of bisoprolol, which includes a backing; and a pressure-sensitive adhesive layer containing bisoprolol, which is laminated on one surface of the backing, wherein the maximum value of a release rate of bisoprolol during a period of from immediately after the application on skin until a lapse of 24 hours is 30 μg / cm2 / hr or less; and wherein the release rate of bisoprolol at the time of a lapse of 24 hours after the application on skin is 10 μg / cm2 / hr or less. The percutaneous administration device of the present invention is reduced in the skin irritation during the application, especially at the time of peeling, and is capable of persistently administrating a therapeutically or preventively effective amount of bisoprolol into a living body.
Owner:NITTO DENKO CORP +1
Ophthalmic composition
ActiveUS20120108658A1Corneal disorder treatmentWithout eye irritationBiocideSenses disorderRapeseedLanolin
Disclosed is an ophthalmic composition which is characterized by containing (A) a vitamin A, (B) a polyoxyethylene polyoxypropylene glycol, and (C) an oil component that is selected from the group consisting of castor oil, soybean oil, sesame oil, peanut oil, olive oil, almond oil, wheat germ oil, corn oil, rapeseed oil, sunflower oil, purified lanolin and gel hydrocarbon.
Owner:LION CORP
Percutaneous absorption preparation comprising Anti-dementia drug
InactiveUS20120282303A1Efficient transdermal administrationSuppressing skin irritationBiocideNervous disorderIrritationPercutaneous absorption
The present invention relates to a percutaneous absorption preparation that is lower skin irritation and enables efficient transdermal administration of an anti-dementia drug. More specifically, the present invention relates to a percutaneous absorption preparation comprising an anti-dementia drug, a polymer compound having an amino group, a polyvalent carboxylate ester, a fatty acid alkyl ester, a styrenic polymer compound, and a tackifier resin.
Owner:TAKESHI GOTO
Transdermal colloidal solution agent
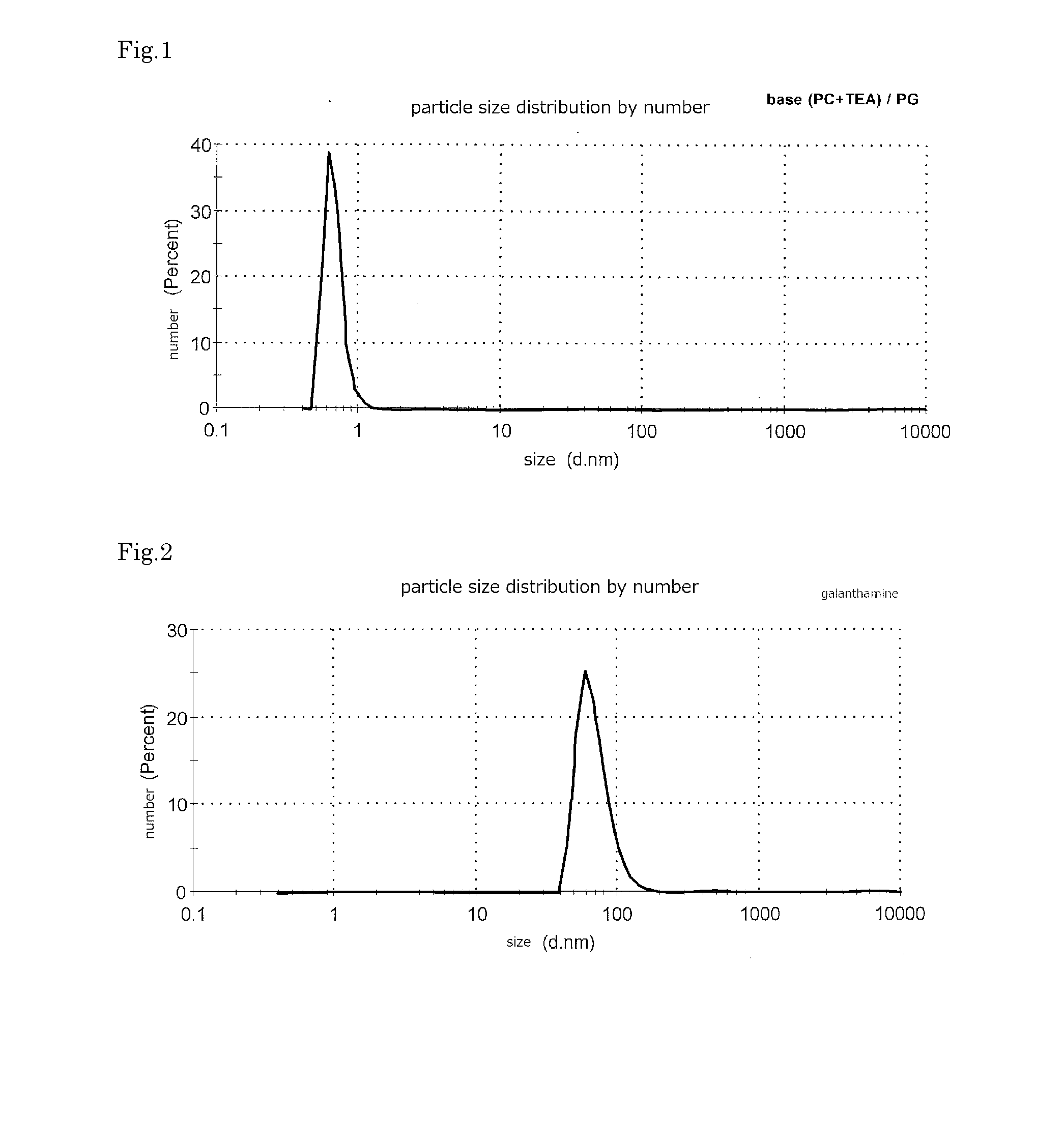
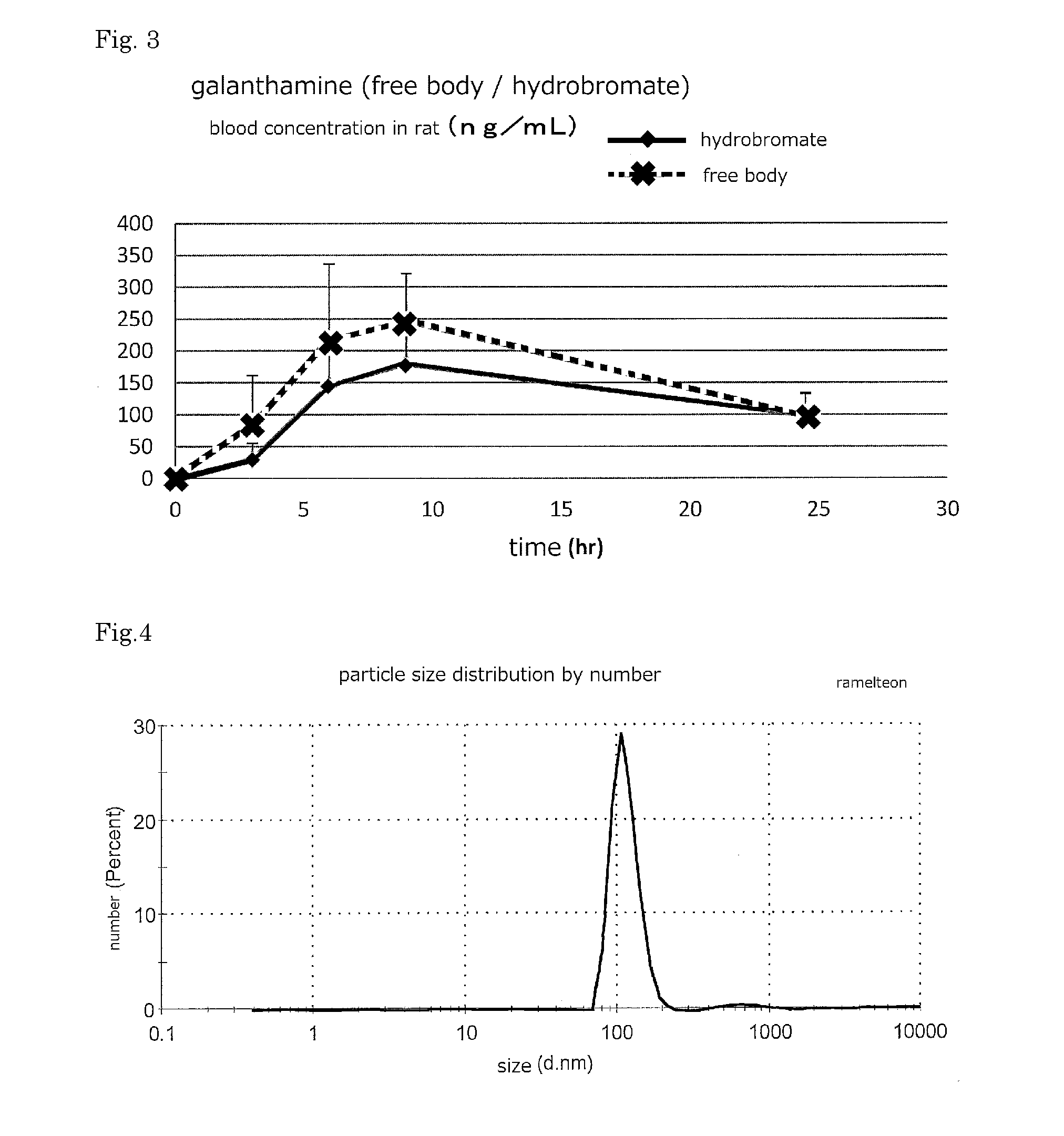
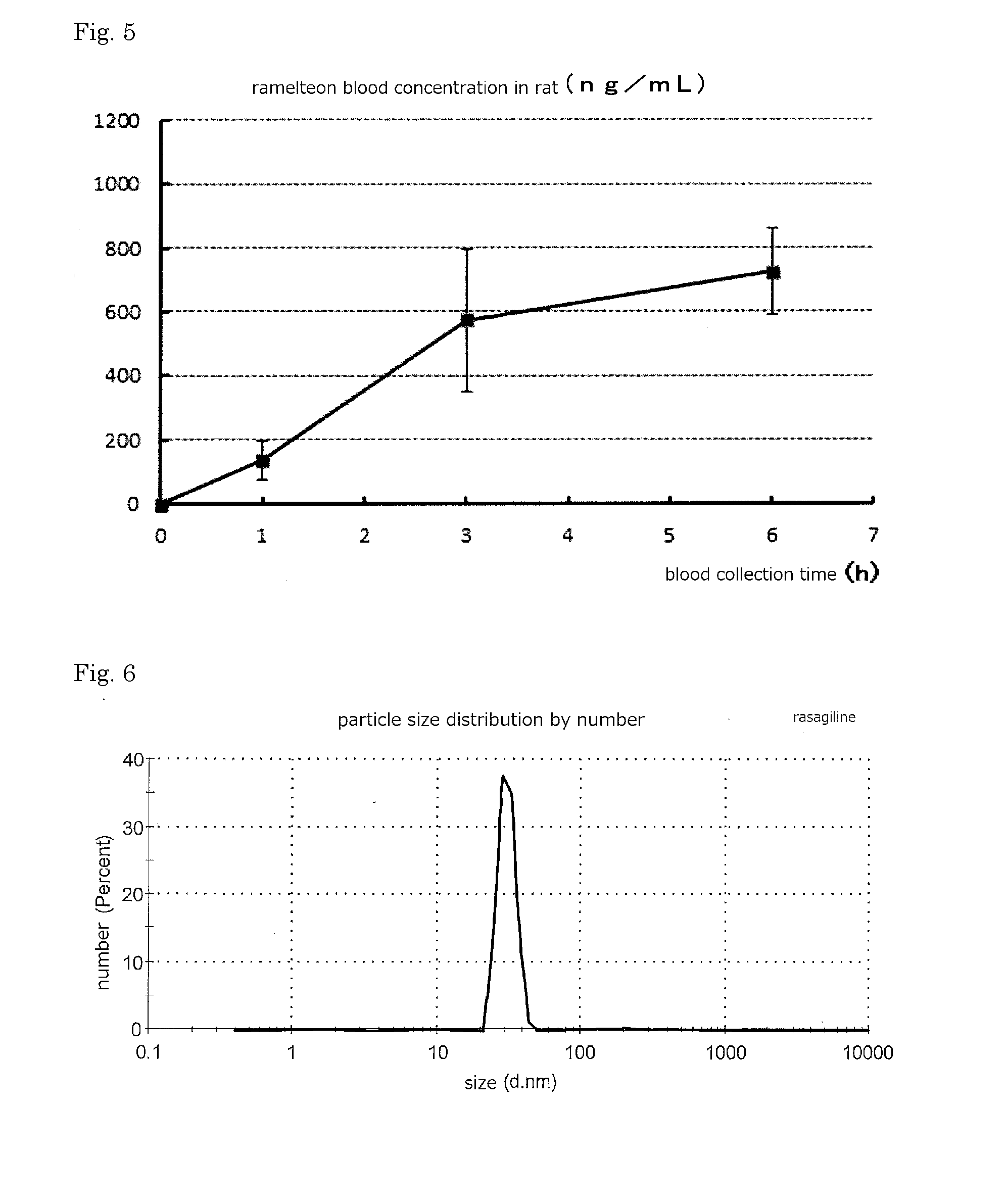
ActiveUS20160256552A1Improves transdermal penetrationFast and persistent permeabilitySolution deliveryPharmaceutical non-active ingredientsSolventPropylene glycol
Disclosed is a transdermal absorptive liquid preparation in which a medicament or a salt thereof is colloidally dispersed in propylene glycol or a propylene glycol-containing solvent, whose transdermal permeability of the medicament is excellent, problem of skin irritation is reduced. This transdermal absorptive liquid formulation has a mode of particle diameter at around 100 nm, and an average particle size of 50 to 500 nm. This transdermal absorptive liquid formulation makes marked improvement in the transdermal permeability by further containing an absorption promoter such as triethanolamine.
Owner:MEDRX CO LTD
Topical two step polytherapy for treatment of psoriasis and other skin disorders
InactiveUS8524774B1Irritation is reduced and controlledMinimize irritationBiocideOintment deliveryAntioxidantCombined Modality Therapy
A process for treating psoriasis and similar skin disorders whereby two formulations are used. The first formulation in a cream, gel, lotion, foam or ointment base is used two or more times daily, and contains effective amounts of (but not limited to) methylcobalamin, niacinamide, select cetylated fatty esters, and antioxidants. This formulation is used to suppress immunoproliferative and inflammatory mediated activities that play significant roles in hyperproliferation and erythema. The second formulation, used selectively to reduce plaque thickening and to reduce irritation, contains effective amounts of (but not limited to) salicylic acid and select cetylated fatty esters in a cream, gel, lotion or ointment base, with pH optimized for exfoliation properties. The unique combination of salicylic acid (or other β- or α-hydroxy acid) and cetylated fatty esters provides keratolytic activity while suppressing irritation and inflammation. The invention for this novel two-step polytherapy, are disclosed including the mode of sequential administration.
Owner:CYMBIOTICS
Colored cigarette paper and preparation method thereof
InactiveCN111074684ASuppress irritationGood flue gas compatibilitySpecial paperPaper coatingBiotechnologyPyrazine
The colored cigarette paper comprises a cigarette paper base material and a coloring material, and is characterized in that the coloring material is melanoidin generated by deep reaction of natural amino acid and reducing sugar; the cigarette paper base material comprises plant fiber and inorganic filler, and the cigarette paper further comprises a combustion improver. Compared with the prior art,the method has the characteristics that macromolecular substance melanin is generated after deep reaction of amino acid and reducing sugar, wherein the substance is dark brown in color and can be used for cigarette paper dyeing. Meanwhile, when the cigarette paper is burnt, pyridine, pyrazine and pyrrole substances generated by pyrolysis of the substances have the effects of inhibiting paper burning gas and irritation of the cigarette paper, and the pyrolysis substances also widely exist in cigarette smoke and have good smoke compatibility. The melanoidin is widely distributed in food, has the characteristics of safety and edibility, and avoids the potential safety hazard caused by the use of synthetic dyes.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Spicy seasoning packet and preparation method thereof
InactiveCN113558215ASuppress irritationRich in aroma substancesFood scienceOff-flavourVANILLA FLAVORING
The invention provides a spicy seasoning packet and a preparation method thereof, and relates to the field of seasoning. The spicy seasoning packet is prepared from the following raw materials: green Chinese onion, onion, garlic, star anise, cardamom, cassia bark, amomum tsao-ko, fennel, bighead atractylodes rhizome, clove, bay leaf, hot pepper, Chinese prickly ash, crushed peanut, hawthorn, sesame seed, thick broad-bean sauce and long pepper. The long pepper, the green Chinese onion and the onion can achieve the effects of removing fishy smell and peculiar smell. Star anise, cardamom, cinnamon, amomum tsao-ko, fennel, bighead atractylodes rhizome, clove, pepper and myrcia provide complex and rich spice fragrance for the whole seasoning bag, hawthorn can stop greasiness, thick broad-bean sauce is used for improving color and flavor, pepper and pepper provide spicy and spicy taste, hawthorn and chopped peanuts can inhibit irritation of pepper to the oral cavity, and it is guaranteed that the seasoning bag is spicy but not irritating.
Owner:黎正林
Ophthalmic composition
InactiveUS9119827B2Corneal disorder treatmentWithout eye irritationBiocideSenses disorderLanolinRapeseed
Disclosed is an ophthalmic composition which is characterized by containing (A) a vitamin A, (B) a polyoxyethylene polyoxypropylene glycol, and (C) an oil component that is selected from the group consisting of castor oil, soybean oil, sesame oil, peanut oil, olive oil, almond oil, wheat germ oil, corn oil, rapeseed oil, sunflower oil, purified lanolin and gel hydrocarbon.
Owner:LION CORP
Anti-wrinkle eye gel and preparation method thereof
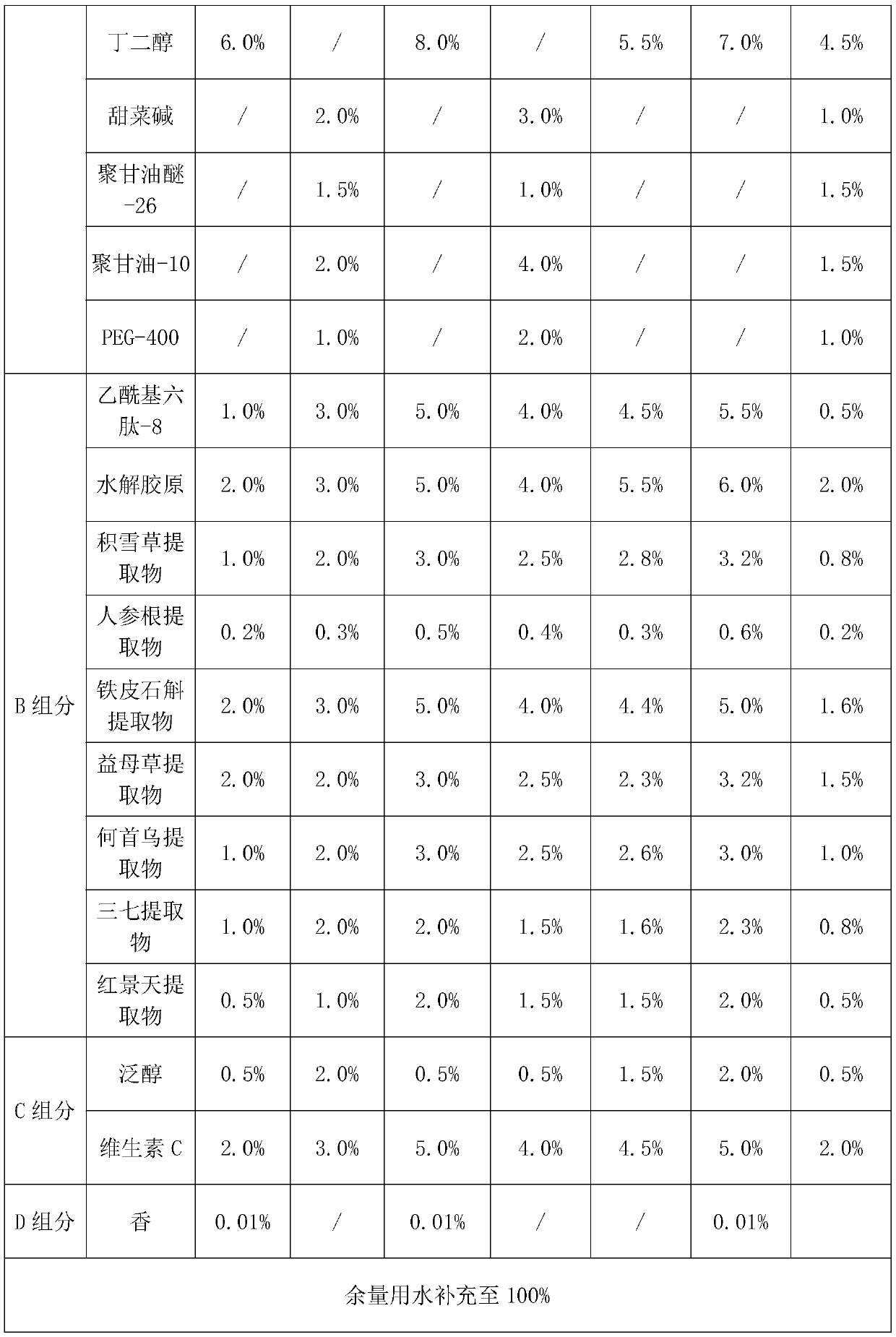
InactiveCN111529469AMild ingredientsReasonable collocationCosmetic preparationsToilet preparationsVitamin CGlycerol
The invention discloses anti-wrinkle eye gel and a preparation method thereof, and belongs to the technical field of cosmetics. The anti-wrinkle eye gel comprises 0.05%-0.3% of xanthan gum, 5.0%-8.0%of glycerol, 1.0%-2.0% of hexanediol, 2.0%-3.0% of pentanediol, 0.05%-0.2% of allantoin, 0.1%-0.5% of sodium hyaluronate, 0.5%-5.0% of acetyl hexapeptide-8, 2.0%-5.0% of hydrolyzed collagen, 0.5%-2.0%of Chinese herbal medicine extract, 0.5%-2.0% of panthenol, 2.0%-5.0% of vitamin C and the balance of deionized water. The preparation method comprises the steps: adding the component A and the deionized water into an emulsifying pot, stirring and homogenizing until the component A and the deionized water are completely dissolved, heating to 85-90 DEG C, keeping the temperature for 30 minutes, cooling to 50 DEG C, adding the component B into the same emulsifying pot, and stirring until the component B is completely dissolved; cooling to 45 DEG C, adding the dissolved component C, uniformly stirring, and continuously cooling; and when the temperature is reduced to 40 DEG C, sampling and performing central control to obtain a finished product. The obtained anti-wrinkle eye gel is mild in component, reasonable in matching, capable of effectively repairing wrinkles and preventing and delaying aging of eye skin, safe and free of irritation.
Owner:广州欧盛化妆品有限公司
Compound sweet zinc cream used for treating eczema of infant and preparation method thereof
InactiveCN102988471AGrowth inhibitionSuppress irritationHydroxy compound active ingredientsInorganic active ingredientsGlycerolGlycerite
The invention relates to an externally applied medicine and in particular to compound sweet zinc cream used for treating eczema of an infant and a preparation method thereof. The compound sweet zinc cream provided by the invention mainly comprises dipotassium glycyrrhizinate, baicalin, zinc oxide, nicotinamide, menthol, vitamin E, glycerin three castor acid polyoxyethylene ester 35 and isopropyl myristate; the preparation method of the compound sweet zinc cream comprises the following steps: respectively taking glycerin three castor acid polyoxyethylene ester 35, isopropyl myristate, vitamin E and menthol to prepare solution A; taking zinc oxide and baicalin, adding a right amount of water at the temperature of 50-60 DEG C to prepare solution B, taking dipotassium glycyrrhizinate and nicotinamide to prepare solution C, adding solution B into solution C, stirring to be uniform, then adding mixed solution into the solution A, stirring for 20-30 minutes, so as to obtain semi-transparent orange emulsion, and split charging, so that the compound sweet zinc cream is obtained. Clinical curative effect observation shows that the effective rate of the compound sweet zinc cream prepared by adopting the preparation method provided by the invention for treating the eczema of the infant can reach up to 91%, and the compound sweet zinc cream can be directly applied to skins and hairs and is good in dispersibility, easy to clean and convenient to use.
Owner:黄石市第一医院
Functional purslane composition and application thereof to daily chemicals
ActiveCN104983650AAvoid dry flakesAlleviate chappedCosmetic preparationsToilet preparationsAllergy reliefActive matter
The invention discloses a functional purslane composition. The functional purslane composition has the rapid allergy relief, restoration and protective screen functions and can effectively improve the condition of the surface of the skin. The functional purslane composition comprises a purslane extract, a high-molecular compound of the phospholipid structure, an amino-acid ester and a solvent capable of being applied to household chemicals. The active matter content of the purslane extract is 0.1%-30.0%. The content of the high-molecular compound of the phospholipid structure is 0.1%-30.0%. The content of glutamate is 0.1%-50.0%. The functional purslane composition can be widely applied to daily chemicals, especially skin care products.
Owner:NANJING HUASHI NEW MATERIAL +1
Health coffee with traditional Chinese medicinal ingredients and preparation method thereof
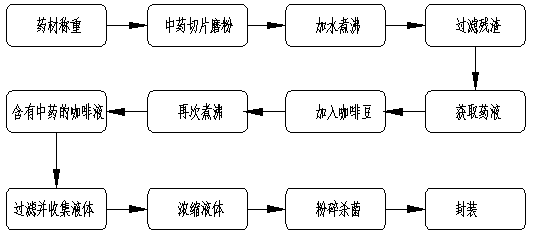
PendingCN111436513AProtect spleen and stomachSupplement the power of bloodRoasted coffee treatmentBiotechnologyGastric digestion
The invention belongs to the technical field of health coffee preparation and particularly relates to health coffee with traditional Chinese medicinal ingredients and a preparation method thereof. Thehealth coffee is prepared from the following raw materials in parts by weight: 60-70 parts of licorice roots, 100-150 parts of ground coffee, 30-50 parts of honey, 160-220 parts of radix rehmanniae praeparata, 100-120 parts of angelica sinensis, 150-200 parts of haws and 50-100 parts of pericarpium citri reticulatae. The preparation method comprises the following steps: grinding the licorice roots, angelica sinensis, haws and pericarpium citri reticulatae into powder; slicing the radix rehmanniae praeparata, boiling the sliced radix rehmanniae praeparata with water, filtering out residues, and extracting medicinal liquid; adding coffee beans into the medicinal liquid, boiling the mixture to obtain coffee liquid rich in traditional Chinese medicines; and performing filtration, collecting medicinal liquid, performing concentration, air-drying, sterilization, package and storage. The health coffee has the refreshing and leisure characteristics of original coffee drinks, has functions oftreating and preventing diseases, has the effects of remarkably relieving fatigue, inhibiting the stimulation of coffee on nervous centralis, stomach and heart and blood vessels, improving myocardialanoxia, promoting intestinal and gastric digestion and reducing the situation of poor excretion and emotional instability, and has health protection and maintenance effects.
Owner:龚羽若
Ground negative-pressure oil extraction process and device thereof
InactiveCN107936938ASuppress irritationGood emulsifying effectFluid removalDrilling compositionSalt resistanceAlkane
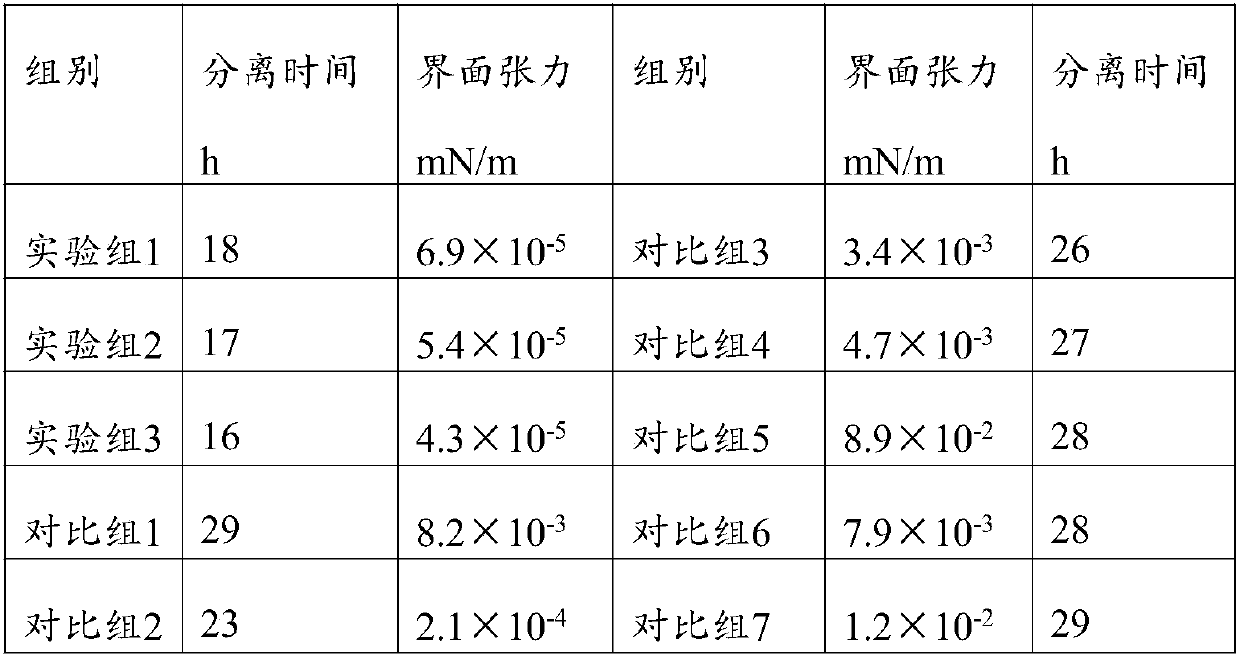
The invention relates to a ground negative-pressure oil extraction process and a device thereof. An oil extraction method comprises introducing a composition as follows into a stratum oil well and extracting oil by using a negative-pressure method; the composition is prepared from a main agent and a synergist, wherein the main agent is prepared from the following components in parts by weight: 15-25 parts of a betaine derivative, 3-7 parts of a glucoside derivative, 10-20 parts of alkane sulfonate, 5-10 parts of heterocyclic salt and 5-10 parts of naphthyl sulfonate; the synergist is preparedfrom at least two of the following components: 2-5 parts of guar gum, 3-5 parts of lignin and 2-5 parts of triethanolamine. Through the ground negative-pressure oil extraction process and the device thereof, the oil is extracted by adding the corresponding composition and the negative-pressure oil extraction process; the effect is obvious; through the interaction between the main agent and the synergist, the oil-water interfacial tension is greatly reduced, and the emulsifying capability is improved; the composition has high salt resistance and high resistance to high temperature, and is capable of resisting the special environment of a stratum and achieving a good oil extraction effect and high oil extraction mount during specific oil extraction.
Owner:张晓静
Composition for enhancing immunity as well as preparation method and application thereof
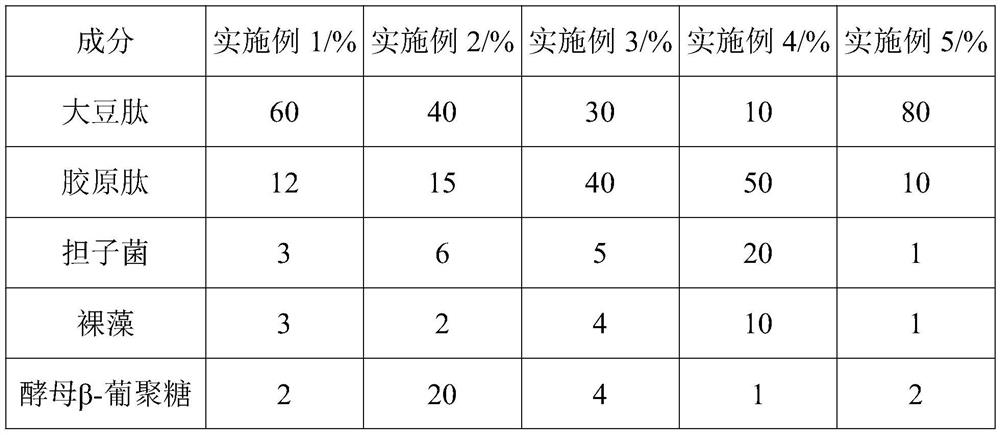
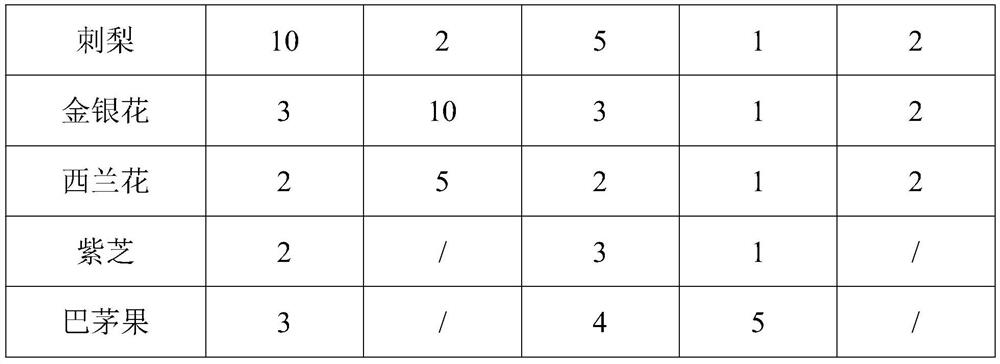
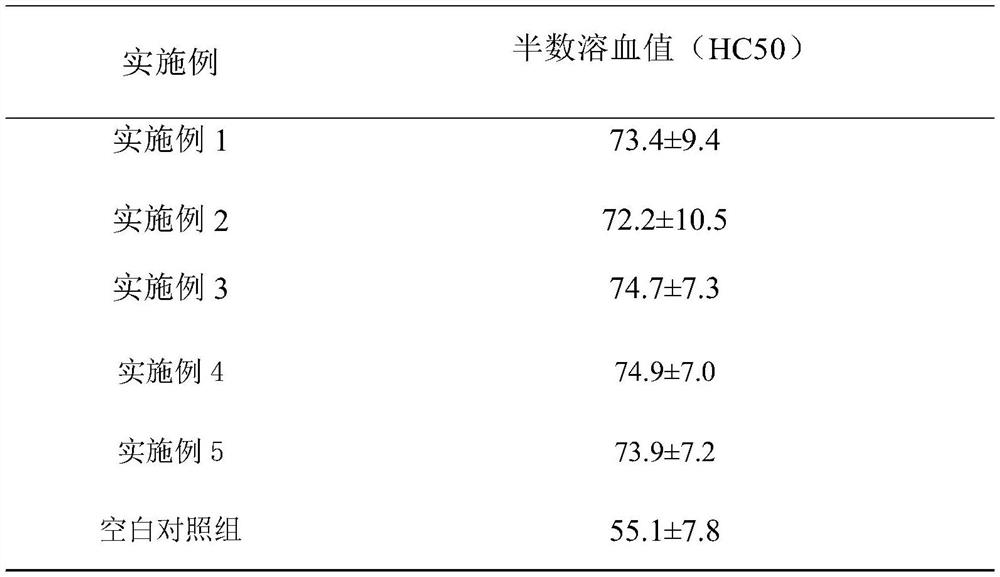
InactiveCN112293740APrevent diseaseFix stability issuesOrganic active ingredientsPeptide/protein ingredientsBiotechnologyImmunocompetence
The invention discloses a composition for enhancing immunity as well as a preparation method and application thereof, and belongs to the technical field of health care products and medicines. The composition for enhancing immunity comprises a component A and a component B, wherein the component A comprises the following substances in percentage by weight: 1-20% of basidiomycetes, 1-10% of euglena,1-20% of yeast beta-glucan, 1-10% of roxburgh rose, 1-10% of honeysuckle flowers and 1-5% of a broccoli seed extract; the component B comprises the following substances in percentage by weight: 10-80% of soybean peptide and 10-50% of collagen peptide; and the component A and the component B are placed into a stirrer to be mixed and stirred, carrying out discharging for later use, and performing mixing and canning to obtain a finished product. The composition can effectively enhance the immunity, and the components are mild and safe; and according to the preparation method of the composition,the activity of all the substances can be maintained, all the components are fully reacted, the overall efficacy and effect are synergistically improved, the irritation of the components is reduced, and the effects of mildness and no irritation are achieved.
Owner:中慈保健品科技开发有限公司
Percutaneous absorption preparation comprising Anti-dementia drug
InactiveUS20120283670A1Skin irritation can be suppressedReduce skin irritationBiocideNervous disorderAlcoholIrritation
The present invention relates to a percutaneous absorption preparation comprising an anti-dementia drug, which is lower skin irritation. More specifically, the present invention relates to a percutaneous absorption preparation comprising a drug-containing layer comprising an anti-dementia drug, a polymer compound having an amino group, a polyhydric alcohol fatty acid ester, a polyhydric alcohol, a polyvalent carboxylate ester, and a styrenic polymer compound, wherein the content of the anti-dementia drug is 0.5-20 mass % of the drug-containing layer.
Owner:TAKESHI GOTO
Efficient mothproof camphor tablet with mildew-proof function
PendingCN114794100ASoft skinImprove itchy skinBiocidePest repellentsSilicone fluidDimethyl formamide
The invention discloses a high-efficiency camphor mothproof tablet with a mildew-proof function, and particularly relates to the technical field of mothproof tablets, and the high-efficiency camphor mothproof tablet is prepared from the following raw materials in percentage by weight: 90-94% of camphor, 0.12-0.15% of sandalwood powder, 0.3-0.6% of potassium sorbate, 0.12-0.25% of glycerol, 0.15-0.55% of phenyl salicylate, 0.01-0.05% of N, N-dimethyl formamide, 0.01-0.05% of magnesium stearate and the balance of water. The cleaning agent is prepared from the following components in percentage by weight: 0.24 to 0.28 percent of nickel N, N-di-n-butyl dithiocarbamate, 0.24 to 0.28 percent of fatty alcohol-polyoxyethylene ether, 0.01 to 0.04 percent of sorbic acid, 0.43 to 0.56 percent of fructus kochiae powder, 0.18 to 0.2 percent of silicone oil, 0.25 to 0.45 percent of alkylolamide, 0.04 to 0.2 percent of allicin powder, 0.1 to 0.3 percent of sophocarpidine, 0.3 to 0.7 percent of cortex meliae powder, 0.02 to 0.04 percent of betel nut powder, 0.12 to 0.15 percent of radix stemonae and the balance of water. By adding the sandalwood powder, skin can be softened, the phenomenon of itching or inflammation of the skin can be improved, the stimulation effect of camphor on mucous membranes and the skin is inhibited, no mildew spot is generated, and the good mildew-proof effect of the camphor is reserved.
Owner:佛山市南海添惠日化有限公司
A kind of aloe antibacterial gel and preparation method thereof
ActiveCN106237029BReduce bleedingWon't breakAntibacterial agentsOrganic active ingredientsChlorhexidine AcetateMedicine
The invention provides an aloe anti-bacterial gel. The aloe anti-bacterial gel is prepared from the following components in percentage by weight: 0.2-10 % of hydroxypropyl methylcellulose, 1-10 % of propylene glycol, 0.1-3 % of chlorhexidine acetate, 0.01-1% of hyaluronic acid, 1-10 % of aloe extracting solution, 0.5-5 % of lanatechead saussurea herb extract, 0.5-5 % of radix arnebiae seu lithospermi extract, and the balance of water, wherein before mixing, the hyaluronic acid, the lanatechead saussurea herb extract and the radix arnebiae seu lithospermi extract are independently coated by adopting lipidosome or nanometer microcapsules. The invention further provides a preparation method of the gel. The aloe anti-bacterial gel can diminish inflammation and resist bacteria, and regulate the functions of the female reproductive system, the efficacies of the most effective ingredients of the medicinal materials can be exerted, and the aloe anti-bacterial gel is suitable for being safely used for a long term.
Owner:汪红梅
a dish detergent
InactiveCN104726227BRich foamAvoid the downside of being expensiveAmpholytes/electroneutral surface-active compoundsDetergent compounding agentsAlkaneSulfate
A high-foam mild tableware detergent formula comprises, by mass, 4-10% of alpha-carboxyltridecyldimethyl amine oxide, 4-10% of C12-C14 secondary alkane sulphonate, 10-20% of C12-C14 fatty alcohol polyoxyethylene ether sulfate, 3-5% of fatty alcohol polyoxyethylene ether AEO-9, 2.5% of magnesium sulfate heptahydrate, 2-4% of urea, 0.1% of an essence, and the balance of water. The tableware detergent provided by the invention has a good decontamination effect and strong decontamination ability; and alpha-carboxyltridecyldimethyl amine oxide can substitute a common thickener alkanolamide to realize a thickening effect, can greatly enhance the mildness of common tableware detergents, and makes foams fine and stable.
Owner:JIANGNAN UNIV
Percutaneous administration device of bisoprolol
InactiveUS8703178B2Suppress irritationReduce skin irritationOrganic active ingredientsBiocideBisoprololLiving body
The present invention relates to a percutaneous administration device of bisoprolol, which includes a backing; and a pressure-sensitive adhesive layer containing bisoprolol, which is laminated on one surface of the backing, wherein the maximum value of a release rate of bisoprolol during a period of from immediately after the application on skin until a lapse of 24 hours is 30 μg / cm2 / hr or less; and wherein the release rate of bisoprolol at the time of a lapse of 24 hours after the application on skin is 10 μg / cm2 / hr or less. The percutaneous administration device of the present invention is reduced in the skin irritation during the application, especially at the time of peeling, and is capable of persistently administrating a therapeutically or preventively effective amount of bisoprolol into a living body.
Owner:NITTO DENKO CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com