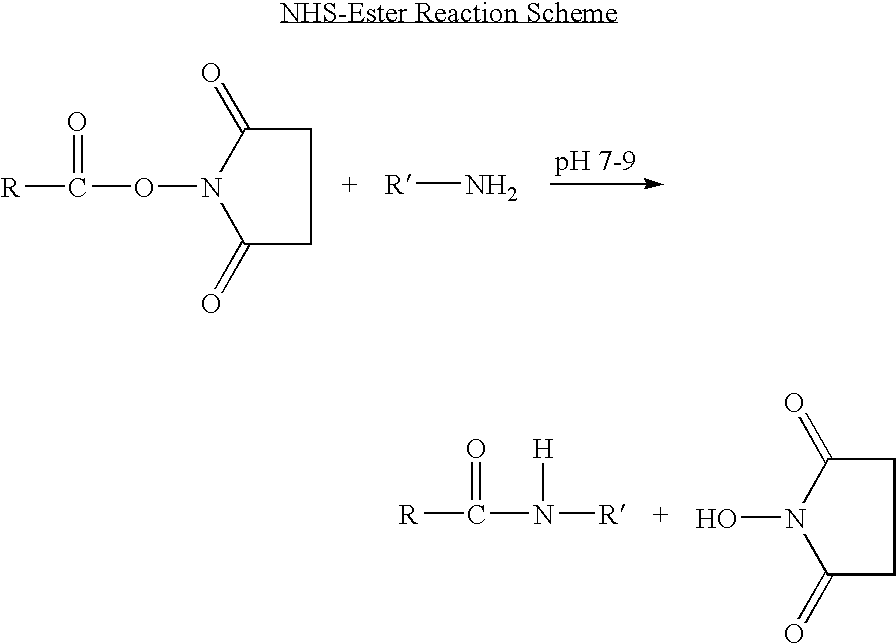
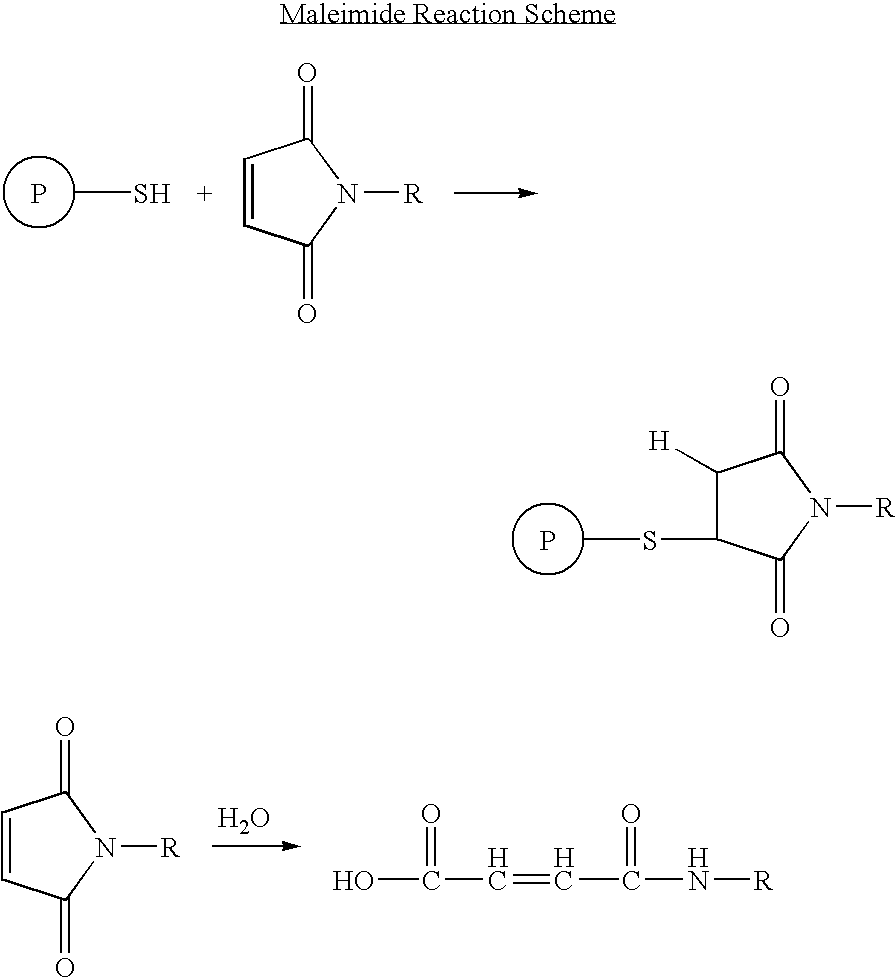
Long lasting fusion peptide inhibitors of viral infection
a technology of fusion peptides and inhibitors, which is applied in the field of modified peptides, can solve the problems of short plasma half-life of peptides and reduce the effective antiviral activity of peptides, and achieve the effects of increasing stability in vivo, and reducing susceptibility to peptidase or protease degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
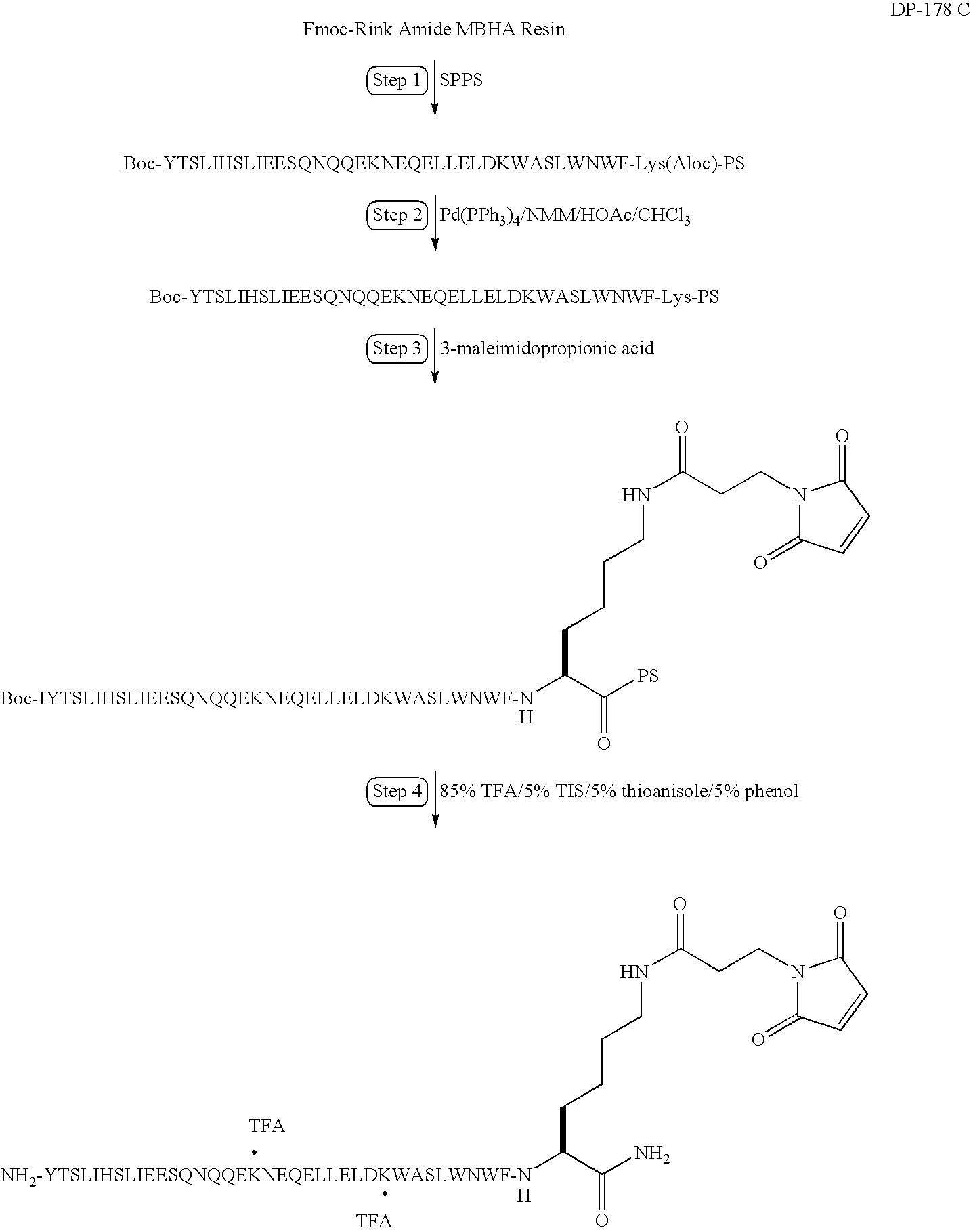
Preparation of a Modified DP178—Synthesis of YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWFK(MPA)-NH2
[0167]In this example, DP178 (SEQ ID NO:1) is synthesized and modified to include a linker and maleimide group according to the following synthesis scheme. As reported in U.S. Pat. Nos. 6,013,236 and 6,020,459, DP178 is a potent inhibitor of HIV-1, and inhibits both cell-induced syncytia formation between HIV-1 infected and uninfected cells and infection of uninfected cells be cell-free HIV-1 virus.
[0168]Solid phase peptide synthesis of the modified peptide on a 100 μmole scale is performed using manual solid-phase synthesis, a Symphony Peptide Synthesizer and Fmoc protected Rink Amide MBHA. The following protected amino acids are sequentially added to resin: Fmoc-Lys(Aloc)-OH, Fmoc-Phe-OH, Fmoc-Trp(Boc)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Trp(Boc)-OH, Fmoc-Leu-OH, Fmoc-Ser(tBu)-OH, Fmoc-Ala-OH, Fmoc-Trp(Boc)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Asp(tBu)-OH, Fmoc-Leu-OH, Fmoc-Glu(tBu)-OH, Fmoc-Leu-OH, Fmoc-Leu-...
example 2
Preparation of a Modified DP107—Synthesis of
[0169]
NNLLRAIEAQQHLLQLTVWQIKQLQARILAVERYLKDQK(MPA)NH2
[0170]In this example, DP107 (SEQ ID NO:2) is synthesized and modified to include a linker and maleimide group according to the following synthesis scheme. As reported in U.S. Pat. Nos. 6,013,236 and 6,020,459, DP107 exhibits potent antiviral activity against HIV.
[0171]Solid phase peptide synthesis of the modified peptide on a 100 μmole scale is performed using manual solid-phase synthesis, a Symphony Peptide Synthesizer and Fmoc protected Rink Amide MBHA. The following protected amino acids are sequentially added to resin: Fmoc-Lys(Aloc)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Asp(tBu)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Leu-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Val-OH, Fmoc-Ala-OH, Fmoc-Leu-OH, Fmoc-Ile-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Ala-OH, Fmoc-Gln(Trt)-OH, Fmoc-Leu-OH, Fmoc-Gln(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Ile-OH, Fmoc-Gln(Trt)-OH, Fmoc-Trp(Boc)-OH, Fmoc-Val-OH, Fmoc-Thr(tBu)-OH, ...
example 3
[0172]Preparation of a Modified anti-RSV Peptide (C Terminal)
[0173]In this example, the peptide VITIELSNIKENKCNGAKVKLIKQELDKYKNAV (SEQ ID NO:16) is modified to include a linker and maleimide group according to the synthesis scheme set forth below. As reported in U.S. Pat. Nos. 6,013,236 and 6,020,459, the native sequence (SEQ ID NO.) inhibits viral infection of respiratory syncytial virus (RSV), including inhibiting fusion and syncytia formation between RSV-infected and uninfected Hep-2 cells.
[0174]Solid phase peptide synthesis of the modified peptide on a 100 μmole scale is performed using manual solid-phase synthesis, a Symphony Peptide Synthesizer and Fmoc protected Rink Amide MBHA. The following protected amino acids are sequentially added to resin: Fmoc-Lys(Aloc)-OH, Fmoc-Val-OH, Fmoc-Ala-OH, Fmoc-Asn(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Asp(tBu)-OH, Fmoc-Leu-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com