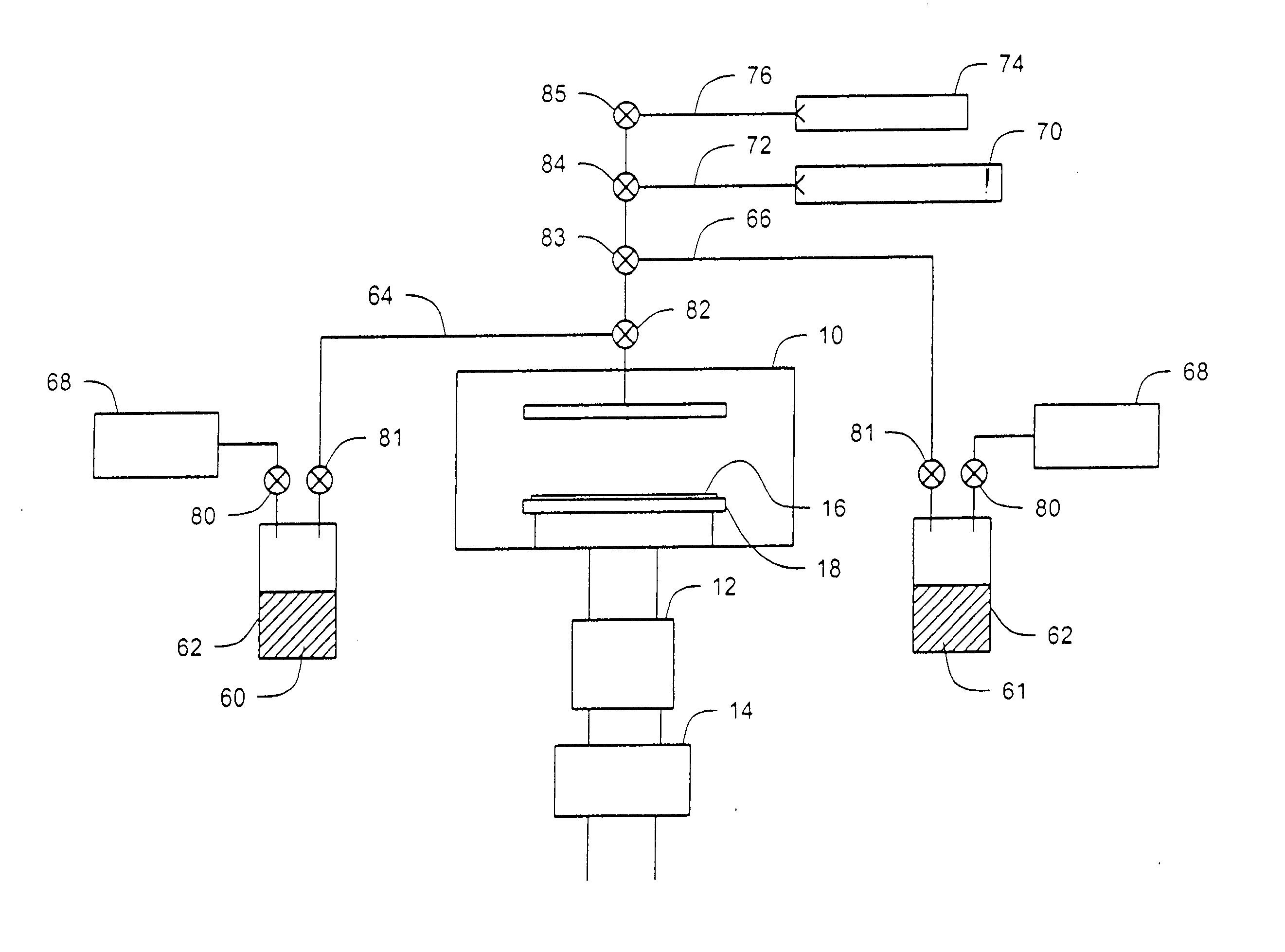
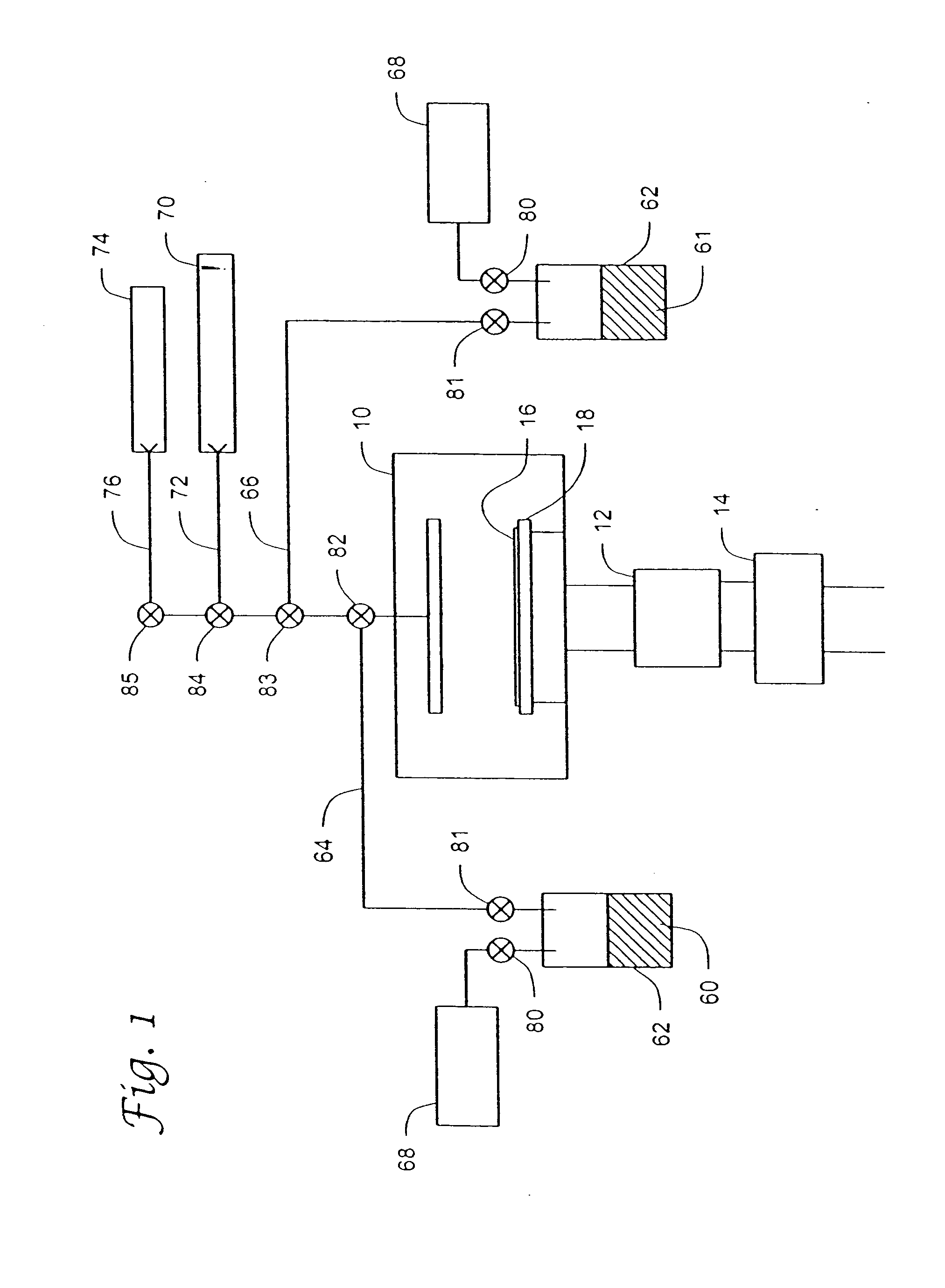
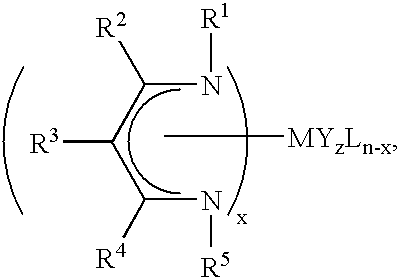
Unsymmetrical ligand sources, reduced symmetry metal-containing compounds, and systems and methods including same
a ligand source and metal-containing compound technology, applied in the field of unsymmetrical ligand sources, reduce symmetry metal-containing compounds, and systems and methods including same, can solve the problems of low thermal stability, poor reactivity of metal diketonates, and difficult integration of alkaline earth metals into vapor deposition processes, so as to minimize harmful gas phase reactions, improve the control of layer thickness and composition, and improve the effect of step coverag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis and Characterization of a Ligand Source of Formula III, with R1=tert-butyl; R5=isopropyl; R2═R4=methyl; and R3═H: N-isopropyl-(4-tert-butylimino)-2-penten-2-amine
[0098] An oven-dry 1-L Schlenk flask was charged with 38.0 g of triethyloxonium tetrafluoroborate (0.2 mol) and 75 mL diethyl ether under argon atmosphere, and fitted with an addition funnel. 250 mL of dichloromethane and 28.2 grams of N-isopropyl-4-amino-3-penten-2-one (0.2 mol) were charged into the addition funnel and this solution was added dropwise, then stirred for 30 minutes. A solution of 21 mL tert-butyl amine (0.2 mol) and 25 mL dichloromethane was charged into the addition funnel and added to the reaction solution, which was then stirred overnight. Volatiles were then removed in vacuo and the resulting yellow-orange solid was washed with two 100 mL aliquots of cold ethyl acetate while the flask was placed in an ice-bath. After decanting off each ethyl acetate wash, the yellow solid residue was added to...
example 2
Synthesis and Characterization of a Metal-containing Compound of Formula I, with M=Sr (n=2); R1=tert-butyl; R5=isopropyl; R2═R4=methyl; R3═H; x=2; and z=0: Strontium bis(N-isopropyl-(4-tert-butylimino)-2-penten-2-aminato)
[0099] In a dry box, a 500 mL Schlenk flask was charged with 13.819 g of strontium bis(hexamethyldisilazane)bis(tetrahydrofuran) (25 mmol) and 100 mL toluene. A second Schlenk flask was charged with 9.800 g of N-isopropyl-(4-tert-butylimino)-2-penten-2-amine (50 mmol) and 100 mL toluene. The ligand solution was added to the strontium solution, immediately producing a bright yellow reaction solution, which was stirred for 60 hours. Volatiles were then removed in vacuo. The crude product, a bright yellow solid, was charged into a sublimator in dry box. The sublimator was attached to a vacuum manifold in a fume hood, evacuated to less than 100 mTorr (13 Pa) and heated to 115° C. A total of 8.204 g of off-white crystalline solid was sublimed in three batches (68.5% yie...
example 3
Synthesis and Characterization of a Metal-containing Compound of Formula II, with M=Sr (n=2); R1═R5=tert-butyl; R6═R10=isopropyl; R2═R4═R7═R9=methyl; R3═R8═H; and z=0: Strontium (N-isopropyl-(4-isopropylimino)-2-penten -2-aminato)(N-tert-butyl-(4-tert-butylimino)-2-penten-2-aminato)
[0100] In a dry box, a 500 mL Schlenk flask was charged with 5.526 g of strontium bis(hexamethyldisilazane) (10 mmol) and 100 mL toluene. A solution of 2.104 g N-tert-butyl-(4-tert-butylimino)-2-penten-2-amine (10 mmol, prepared according to literature) in 20 mL toluene was added to the reaction flask. The reaction solution was stirred for 18 hours. A solution of 1.823 g N-isopropyl-(4-isopropylimino)-2-penten-2-amine (10 mmol, prepared according to literature) in 20 mL toluene was added to the reaction flask. The reaction solution was then stirred an additional 24 hours. Volatiles were removed in vacuo to afford a red-brown solid, which was charged into a sublimator in a dry box (4.70 g, 9.98 mmol). The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dielectric constant | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com