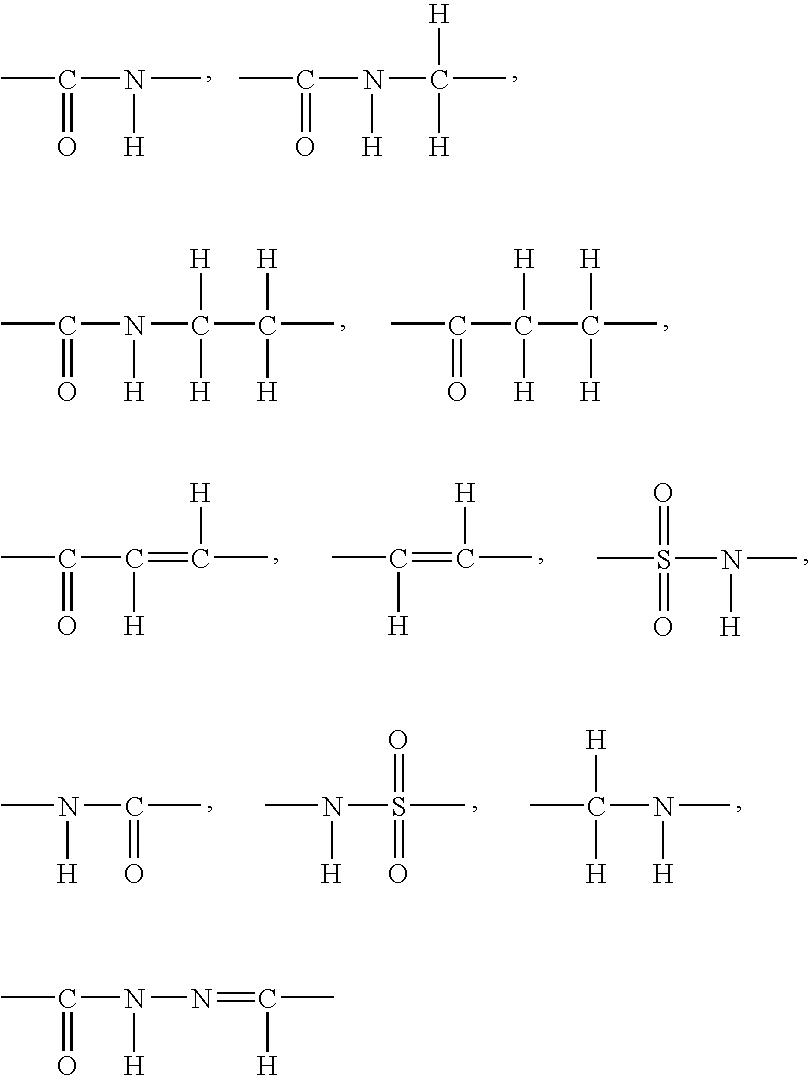Inflammatory cytokine release inhibitor
a cytokine release inhibitor and inflammatory technology, applied in the field of pharmaceutical compositions, can solve the problems of inconvenient long-term use, drug is also aspirin is not suitable for long-term use, so as to avoid side effects and inhibit the effect of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-{[3,5-bis(trifluoromethyl)phenyl]methyl}-5-bromo-2-hydroxybenzamide
Compound No. 1
[0491]Under argon atmosphere, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (it is abbreviated as WSC.HCl hereafter.; 192 mg, 1 mmol) was added to a mixture of 5-bromosalicylic acid (217 mg, 1 mmol), 3,5-bis(trifluoromethyl)benzylamine (243 mg, 1 mmol), 4-dimethylaminopyridine(12 mg, 0.1 mmol) and tetrahydrofuran (10 mL), and the mixture was stirred at room temperature for 1 hour. The reaction mixture was poured into diluted hydrochloric acid and extracted with ethyl acetate. After the organic layer was washed with water and brine, dried over anhydrous magnesium sulfate, the residue obtained by evaporation under reduced pressure was purified by chromatography on silica gel(n-hexane:ethyl acetate=4:1) to give the title compound(244.8 mg, 55.4%) as a white solid.
[0492]1H-NMR (DMSO-d6): δ 4.69 (2H, d, J=5.7 Hz), 6.93 (1H, d, J=8.7 Hz), 7.56 (1H, dd, J=8.7, 2.4 Hz), 8.02 (1H, ...
example 4
3-(1-Chlorophenyl)-1-(2,6-dihydroxyphenyl)-3-(4-hydroxyphenyl)propan-1-one
Compound No. 4
[0501]This compound is a commercially available compound.
Supplier: Apin Chemicals.
[0502]Catalog code number: N 0100D.
example 5
1-(5-Chloro-2-hydroxyphenyl)-3-(4-methoxyphenyl)propan-1-one
Compound No. 5
[0503]This compound is a commercially available compound.
Supplier: Specs.
[0504]Catalog code number: AI-233 / 31581024.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com