Modification of polymers having aromatic groups through formation of boronic ester groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
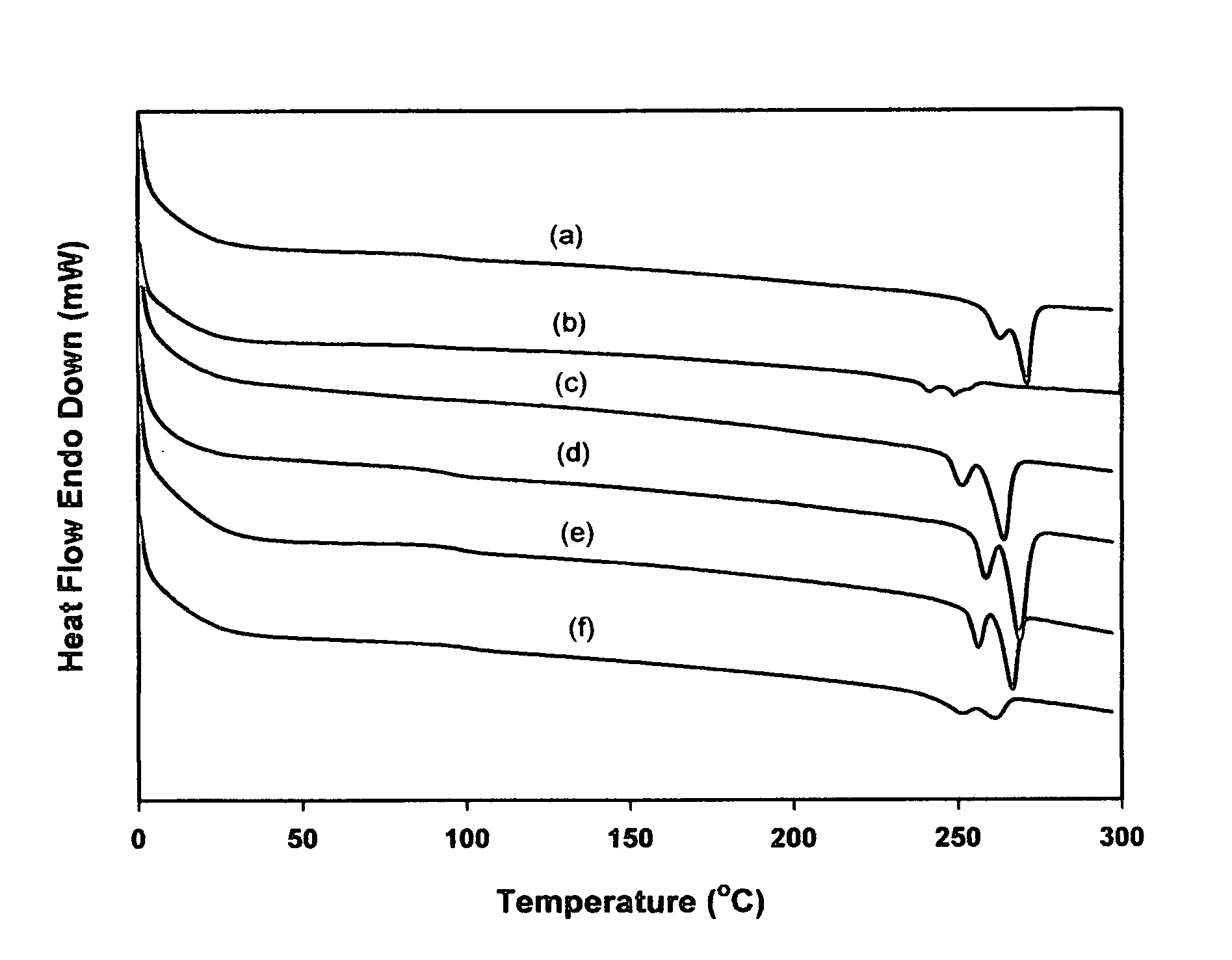
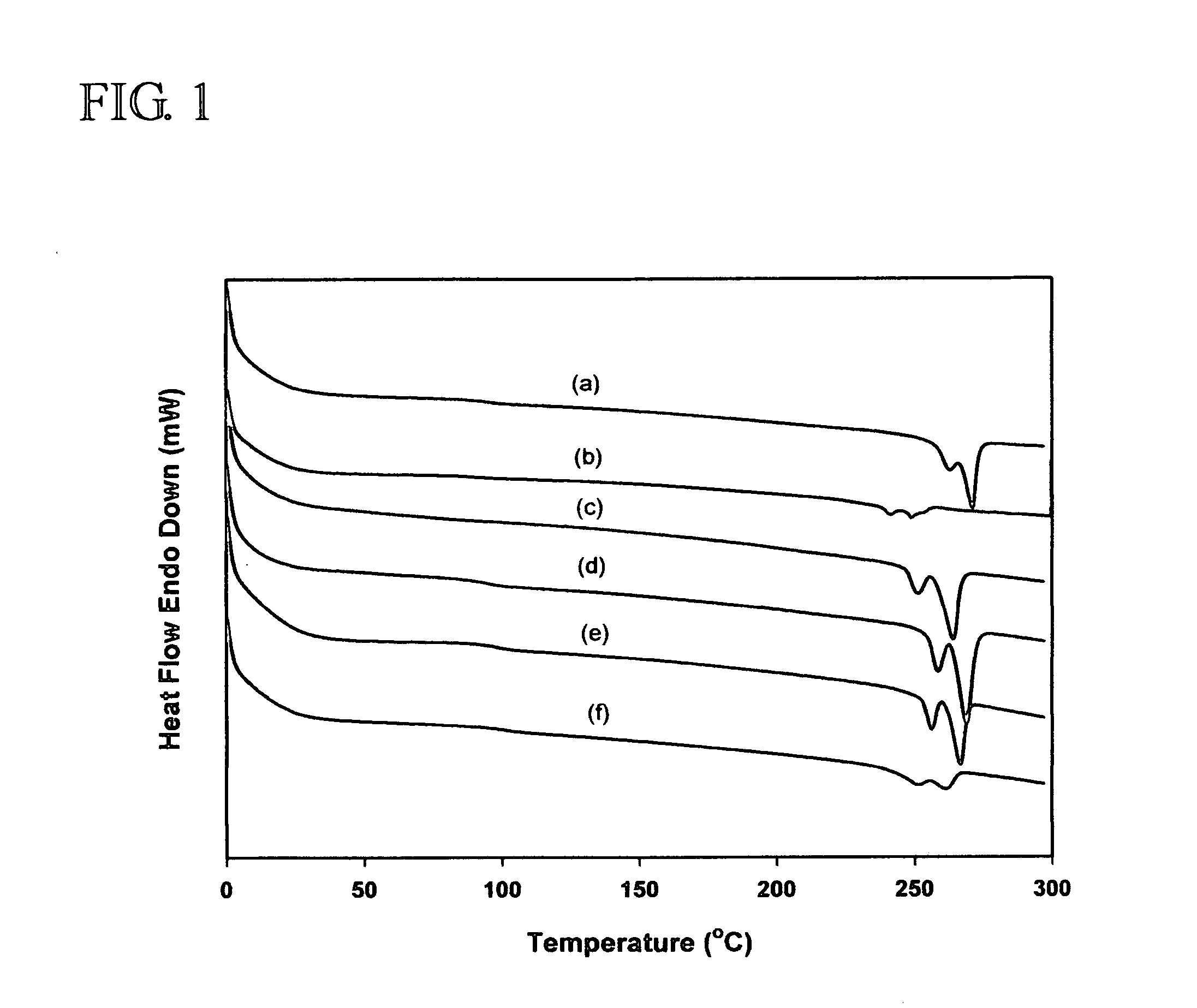
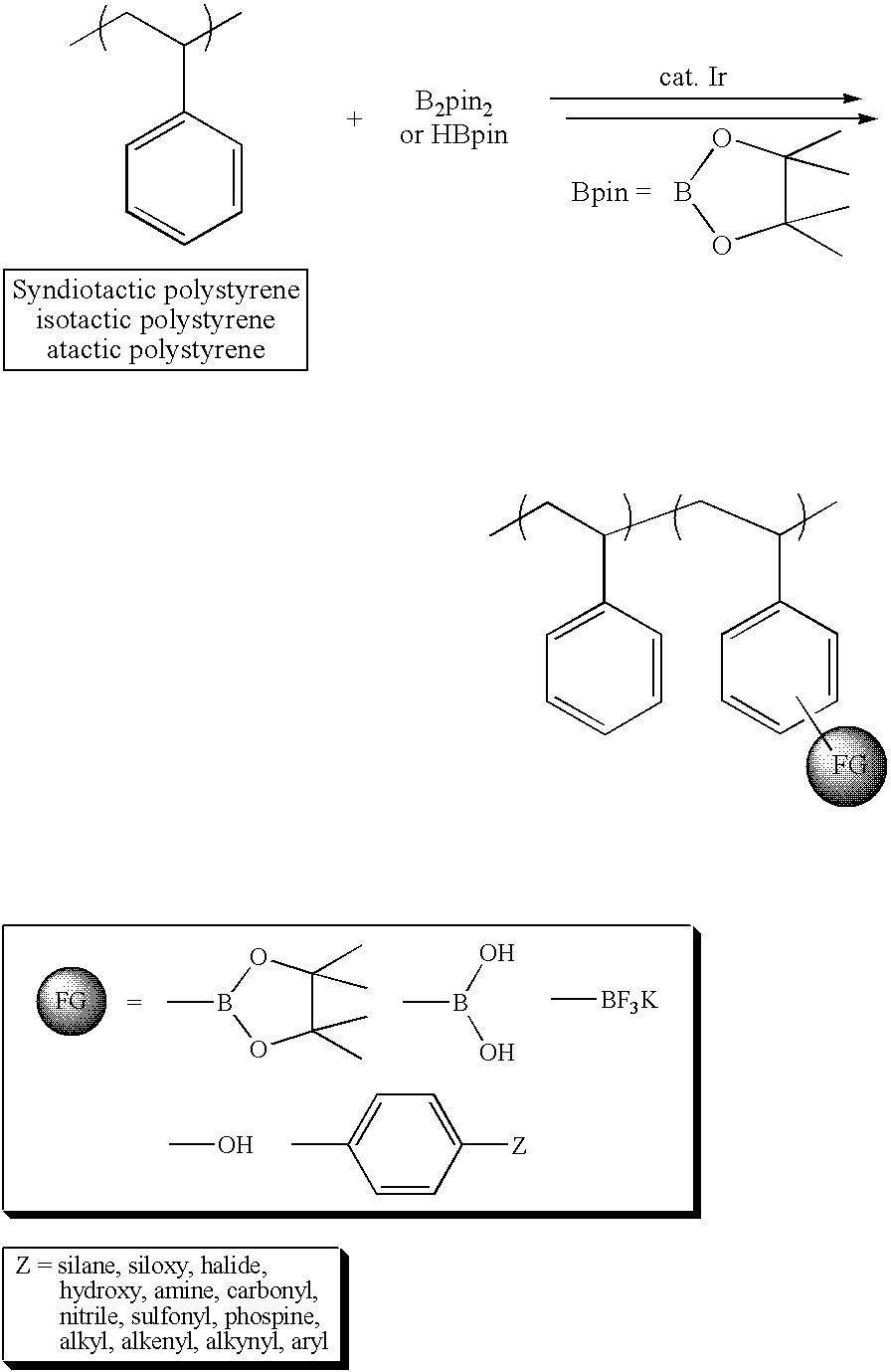
[0017]sPS (Mn=127 kg / mol, PDI=2.64] from LG Chemical Ltd., Daejeon, S. Korea, model atactic polystyrenes of two different molecular weights (Mn=26.3 kg / mol, PDI=1.00) from Aldrich Chemical Co., dtbpy, [IrCl(COD)]2, HBpin, hydrogen peroxide, tetrahydrofuran, sodium hydroxide, and chloroform were reagent grade and used without further purification. B2pin2 was obtained from Frontier Scientific Co. and used after recrystallization from hexane. Cyclooctane was dried using sodium and benzophenone, distilled under reduced pressure, and stored in a nitrogen-filled glove box. To improve the solubility of iPS (Mw=309 kg / mol, 90% isotactic, PDI=6.42 from Aldrich Chemical Co.) in the borylation medium, the following procedure was performed. One gram of the polymer was placed in a two neck round-bottom flask, and then the flask was evacuated and backfilled with nitrogen three times. 1,2-Dichlorobenzene (30 mL) was added to this flask and refluxed at 180° C. under nitrogen for 30 min to dissolve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com