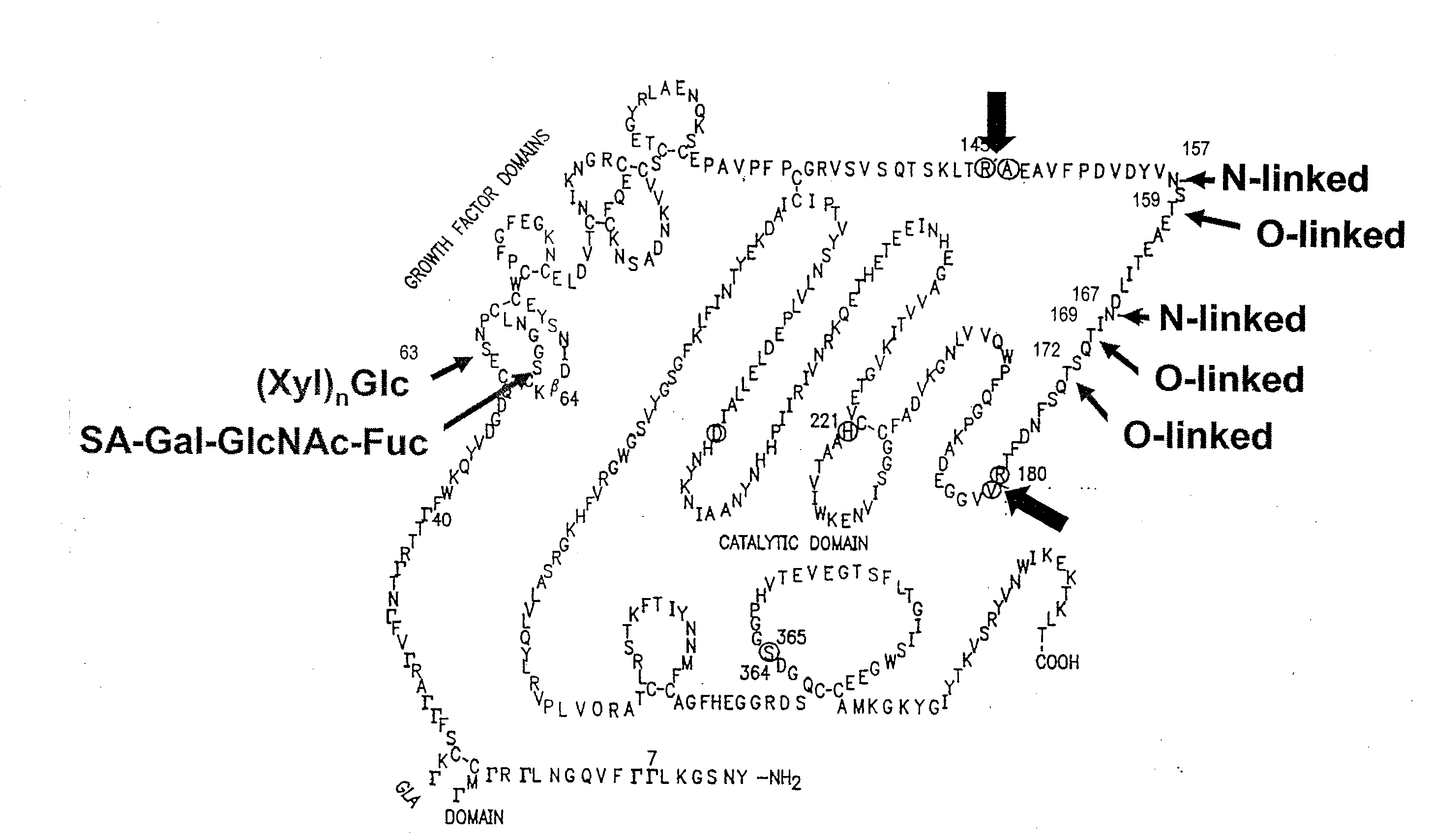
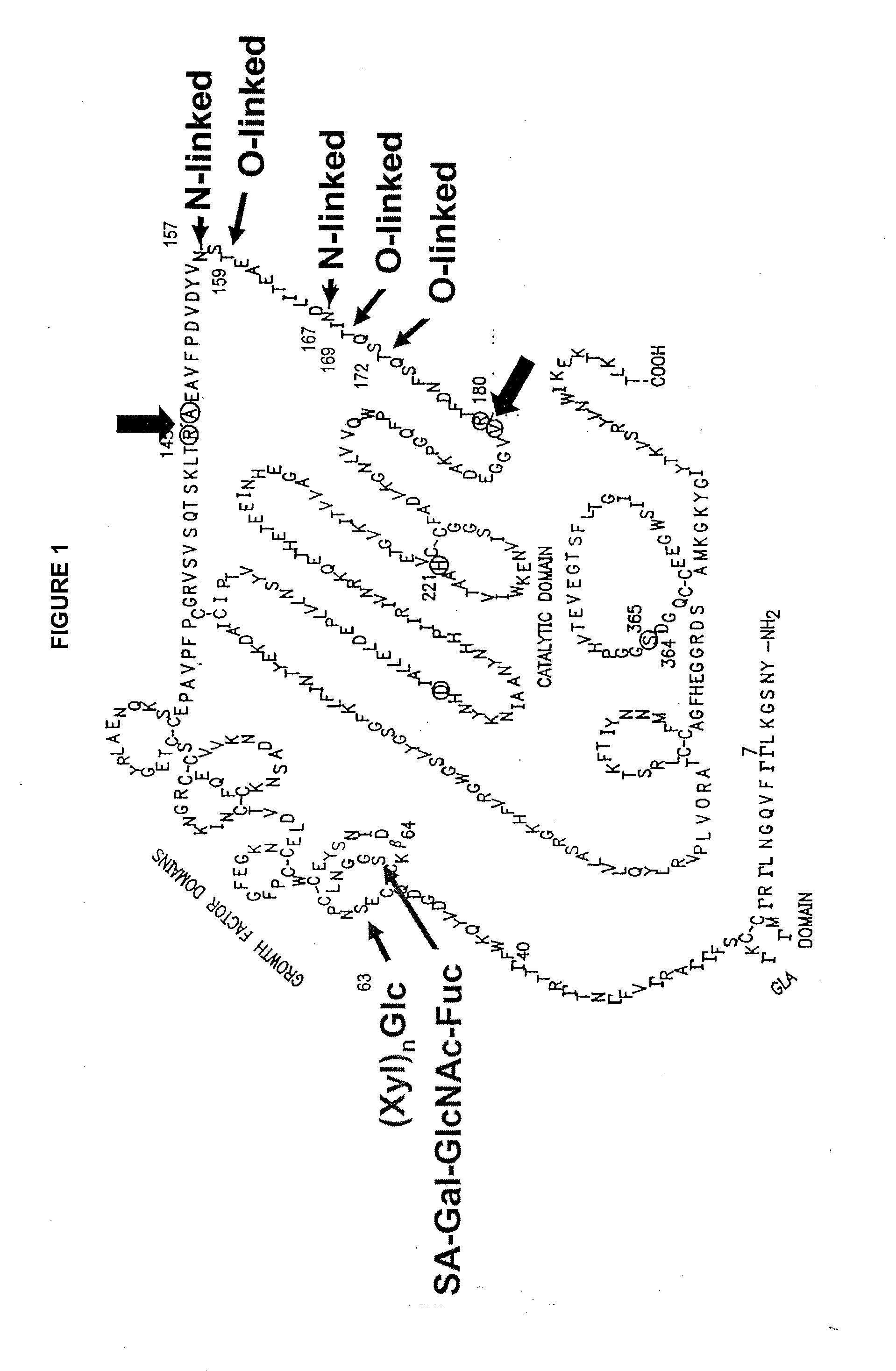
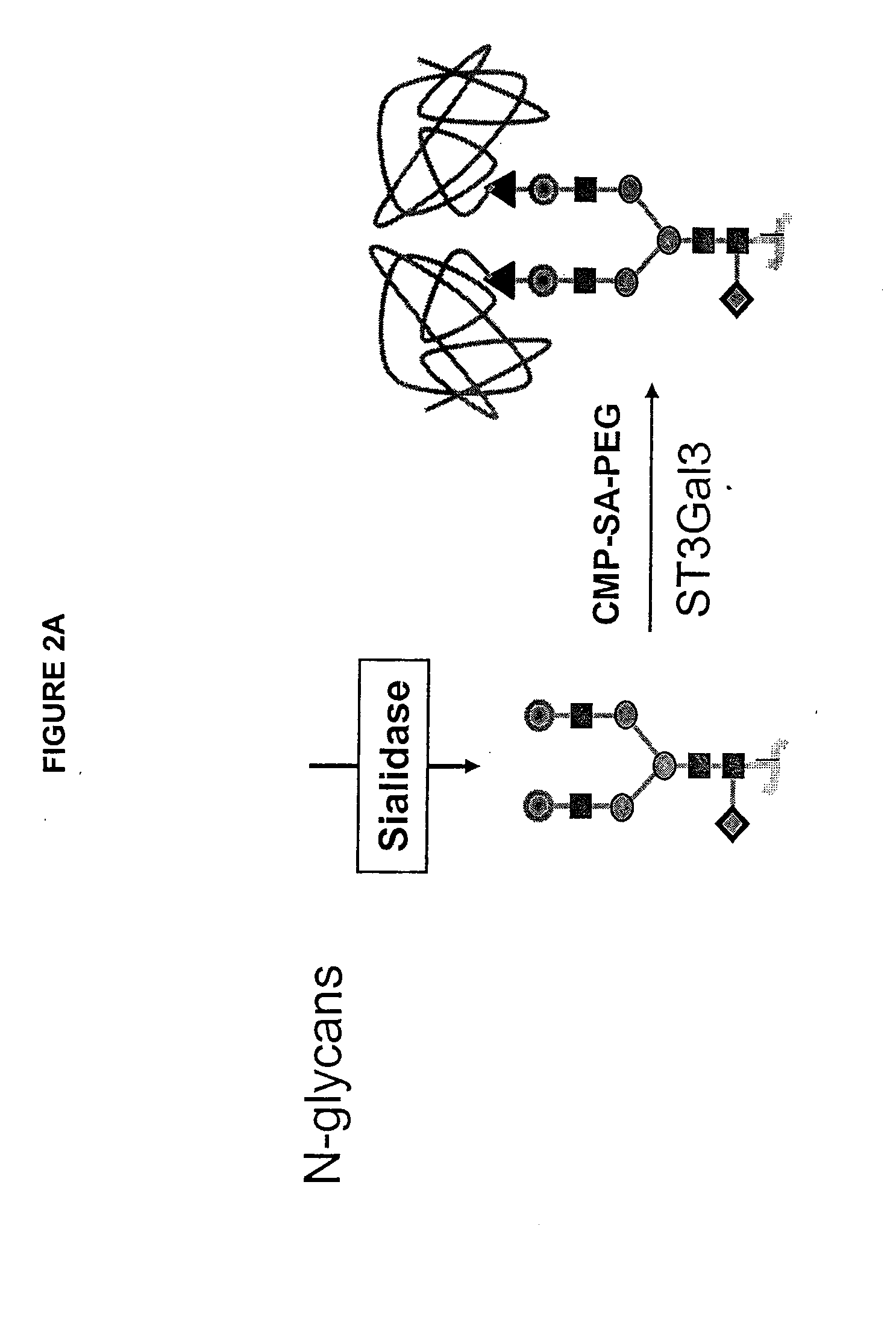
Glycopegylated factor ix
a technology of glycopegylated factor and glycine, which is applied in the direction of enzyme stabilisation, peptide/protein ingredients, extracellular fluid disorder, etc., can solve the problems of inability to form blood clots, formation of unwanted blood clots, and most current therapies have undesirable side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Cysteine-PEG2 (2)
[0339]
1.1 Synthesis of (1)
[0340]Potassium hydroxide (84.2 mg, 1.5 mmol, as a powder) was added to a solution of L-cysteine (93.7 mg, 0.75 mmol) in anhydrous methanol (20 mL) under argon. The mixture was stirred at room temperature for 30 min, and then mPEG-O-tosylate of molecular mass 20 kilodalton (Ts; 1.0 g, 0.05 mmol) was added in several portions over 2 hours. The mixture was stirred at room temperature for 5 days, and concentrated by rotary evaporation. The residue was diluted with water (30 mL), and stirred at room temperature for 2 hours to destroy any excess 20 kilodalton mPEG-O-tosylate. The solution was then neutralized with acetic acid, the pH adjusted to pH 5.0 and loaded onto a reverse phase chromatography (C-18 silica) column. The column was eluted with a gradient of methanol / water (the product elutes at about 70% methanol), product elution monitored by evaporative light scattering, and the appropriate fractions collected and diluted wit...
example 2
GlycoPEGylation of Factor IX Produced in CHO Cells
[0342]This example sets forth the preparation of asialoFactor IX and its sialylation with CMP-sialic acid-PEG.
2. 1 Desialylation of rFactor IX
[0343]A recombinant form of Coagulation Factor IX (rFactor IX) was made in CHO cells. 6000 IU of rFactor IX were dissolved in a total of 12 mL USP H2O. This solution was transferred to a Centricon Plus 20, PL-10 centrifugal filter with another 6 mL USP H2O. The solution was concentrated to 2 mL and then diluted with 15 mL 50 mM Tris-HCl pH 7.4, 0.15 M NaCl, 5 mM CaCl2, 0.05% NaN3 and then reconcentrated. The dilution / concentration was repeated 4 times to effectively change the buffer to a final volume of 3.0 mL. Of this solution, 2.9 mL (about 29 mg of rFactor IX) was transferred to a small plastic tube and to it was added 530 mU α2-3,6,8-Neuraminidase-agarose conjugate (Vibrio cholerae, Calbiochem, 450 μL). The reaction mixture was rotated gently for 26.5 hours at 32° C. The mixture was centri...
example 3
Preparation of PEG (1 kDa and 10 kDa)-SA-Factor IX
[0344]Desialylated rFactor-IX (29 mg, 3 mL) was divided into two 1.5 mL (14.5 mg) samples in two 15 mL centrifuge tubes. Each solution was diluted with 12.67 mL 50 mM Tris-HCl pH 7.4, 0.15 M NaCl, 0.05% NaN3 and either CMP-SA-PEG-1k or 10k (7.25 μmol) was added. The tubes were inverted gently to mix and 2.9 U ST3Gal3 (326 μL) was added (total volume 14.5 mL). The tubes were inverted again and rotated gently for 65 hours at 32° C. The reactions were stopped by freezing at −20° C. 10 μg samples of the reactions were analyzed by SDS-PAGE. The PEGylated proteins were purified on a Toso Haas Biosep G3000SW (21.5×30 cm, 13 um) HPLC column with Dulbecco's Phosphate Buffered Saline, pH 7.1 (Gibco), 6 mL / min. The reaction and purification were monitored using SDS Page and IEF gels. Novex Tris-Glycine 4-20% 1 mm gels were loaded with 10 μL (10 μg) of samples after dilution with 2 μL of 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% NaN3 buffer and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com