Separation of water from hydrocarbons
a technology of hydrocarbon fuel and water, applied in the field of method, can solve the problems of accelerating corrosion, exacerbated problems of product quality control, and excessive amounts of water frequently adversely affecting the properties and quality of hydrocarbon fuels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
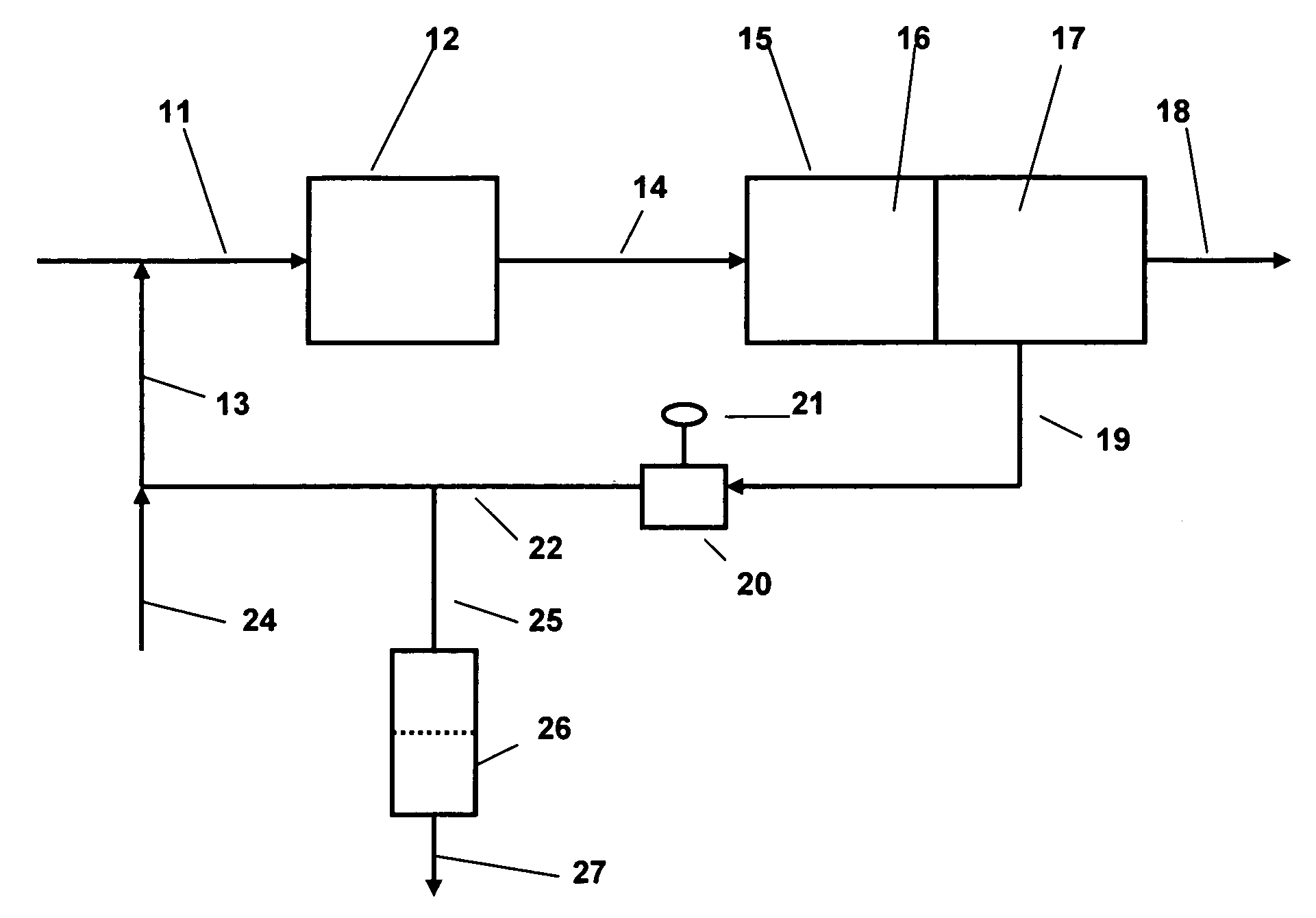
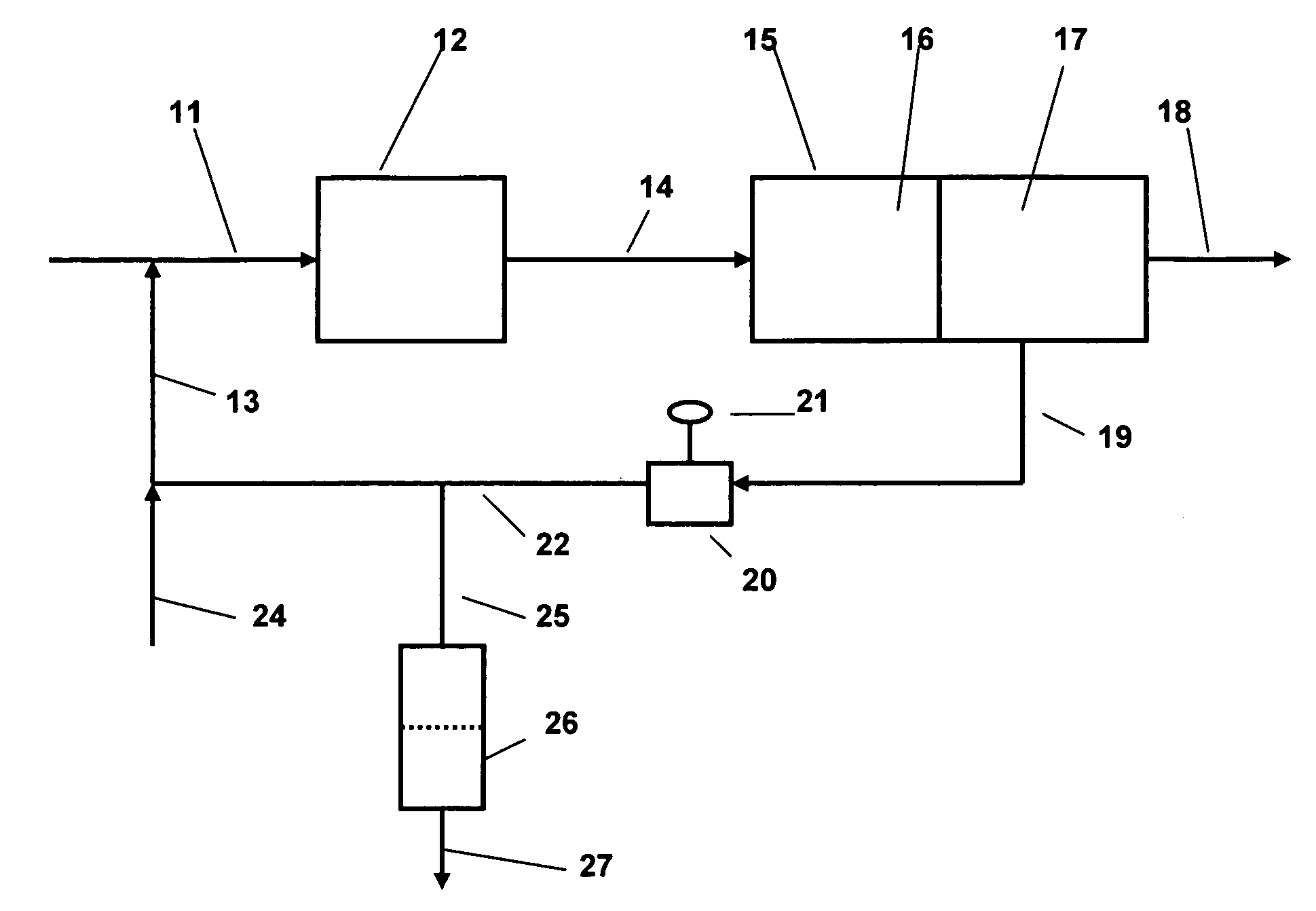
[0015]The present invention is applicable to the separation of water, either alone or mixed with other contaminants, from hydrocarbon liquids. The method is particularly applicable to the separation of water from light hydrocarbon liquids of relatively low viscosity, comparable to that of the water to be separated. The method is of particular applicability to the separation of water from refinery hydrocarbon fuels including gasoline (including heavy gasoline and light gasoline), middle distillates such as home heating oil, vaporizing oil, road diesel including all ASTM D2 diesels, kerosene type aviation fuels, as well as potentially to other liquid hydrocarbon product streams which require removal of water in order to meet product specifications or other service or commercial requirements. Normally, the amount of water which is present in these materials prior to separation will be relatively small, typically not more than about 5 volume percent, but product specifications will norm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com