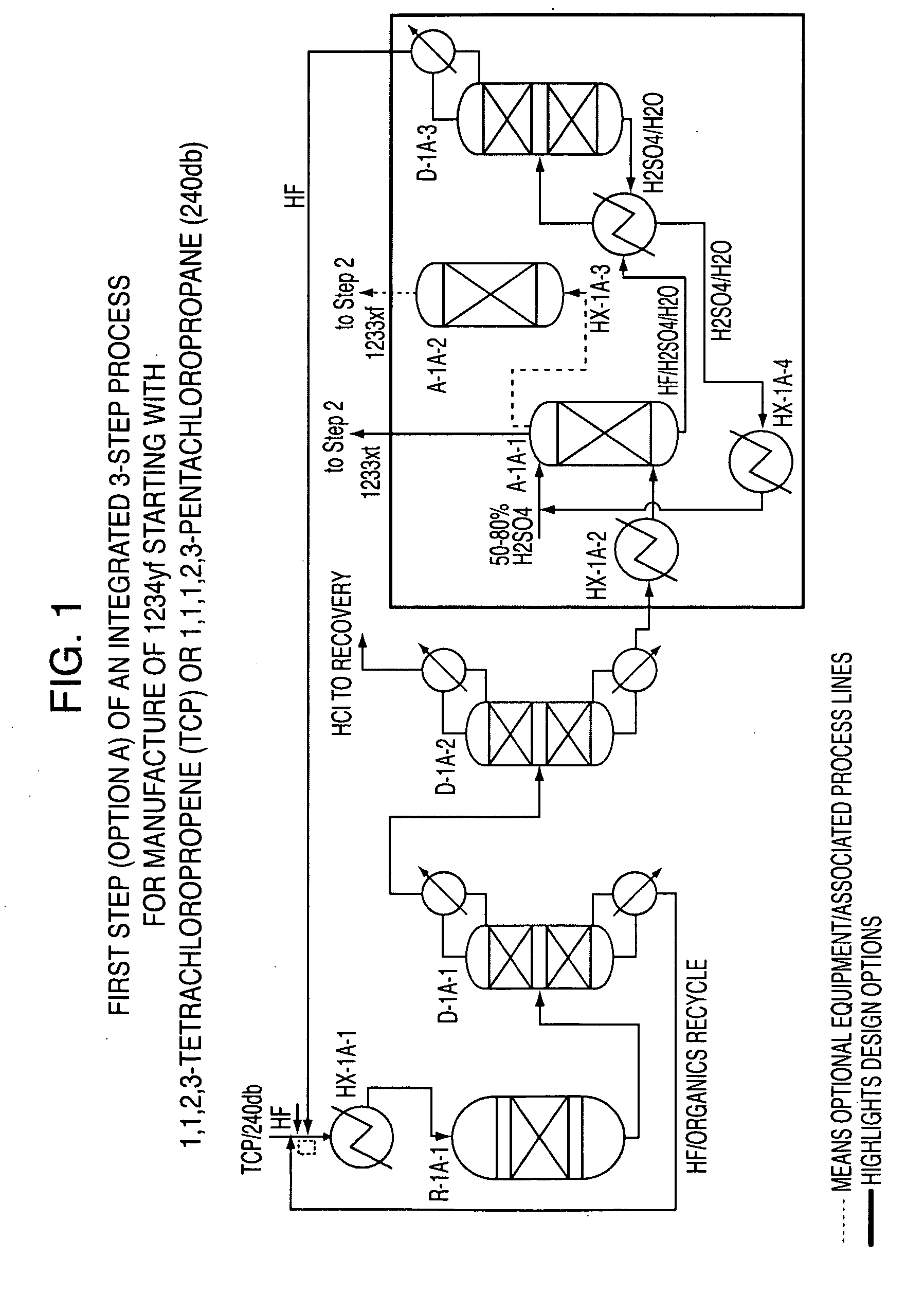
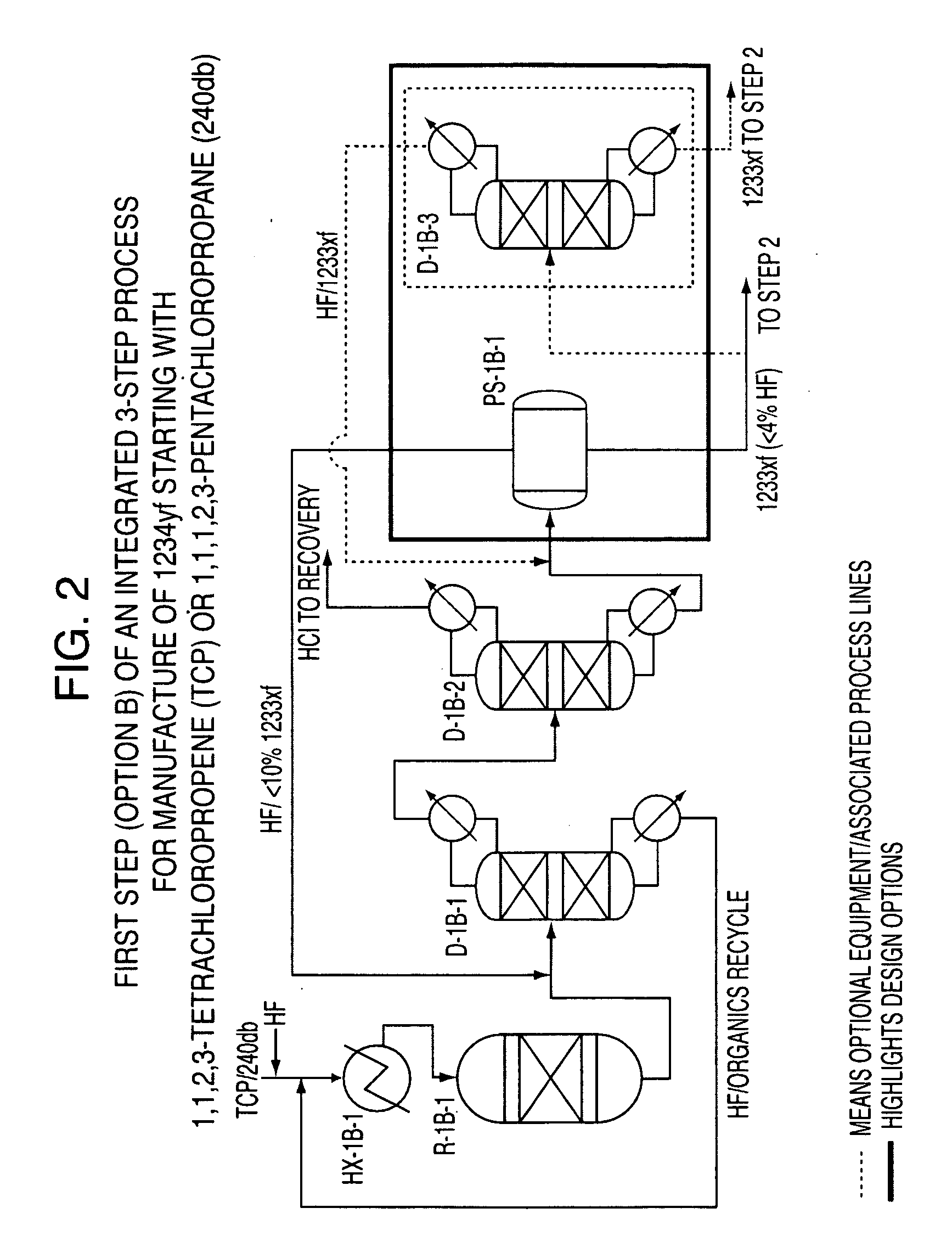
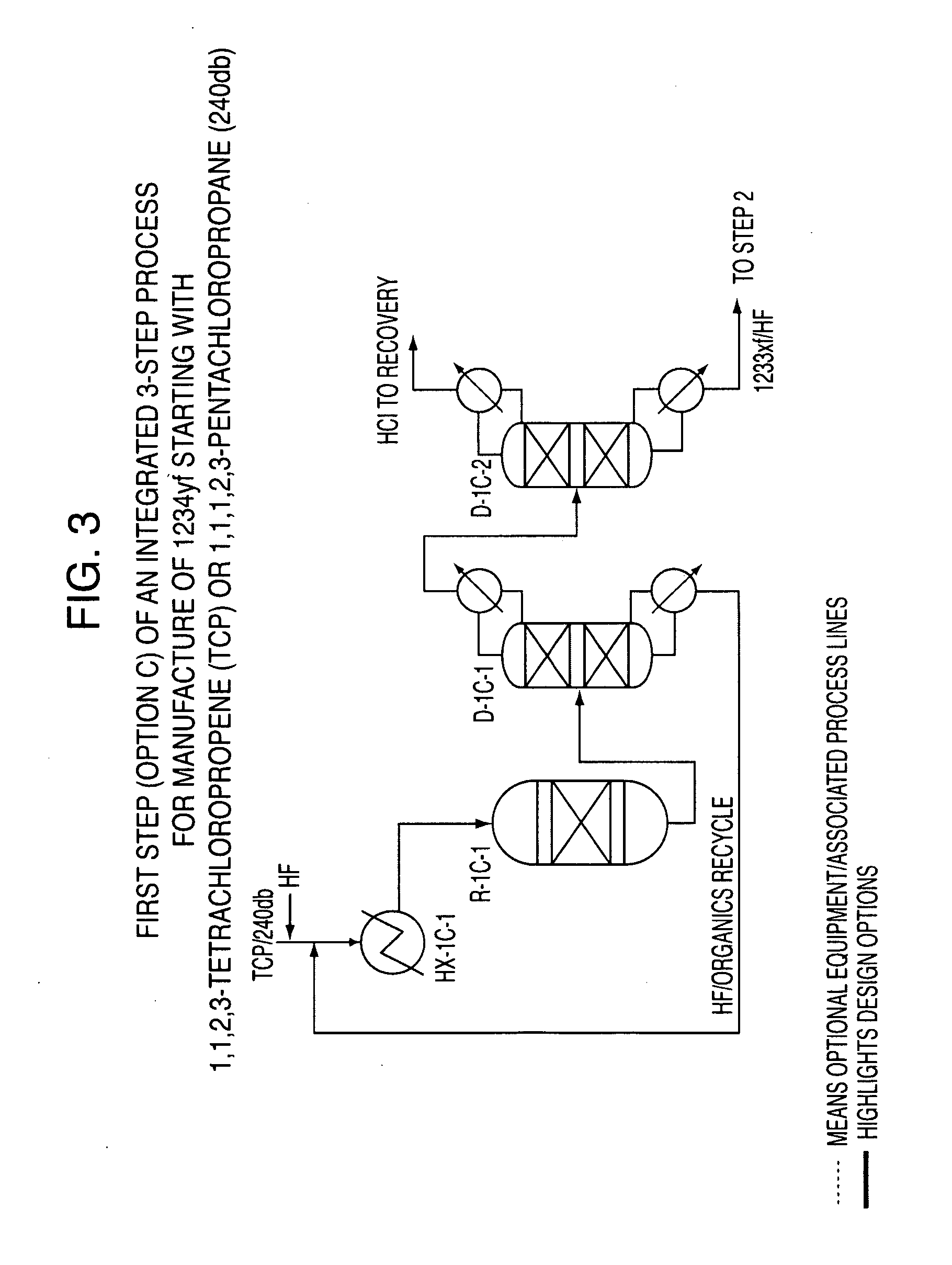
Integrated process to produce 2,3,3,3-tetrafluoropropene
a technology of tetrafluoropropene and integrated process, which is applied in the field of fluorinated organic compound production, can solve the problems of low yield, high cost of commercial hydrogen gas production, and high temperature handling of hydrogen gas at high temperature, so as to maximize raw material utilization and product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0049]This example illustrates Step 1 of the continuous vapor phase fluorination reaction of 1,1,2,3-tetrachloropropene (TCP)+3HF→2-chloro-3,3,3-trifluoropropene (HCFO-1233xf)+3HCl. The fluorination catalyst for the experiment was fluorinated Cr2O3.
[0050]A continuous vapor phase fluorination reaction system consisting of N2, HF, and organic feed systems, feed vaporizer, superheater, 4 inch ID Monel reactor, acid scrubber, drier, and product collection system was used to study the reaction. The reactor was loaded with 9415.2 grams of pretreated Cr2O3 catalyst which equates to about 6.5 liters of catalyst. The reactor was then heated to a reaction temperature of about 235° C. with a N2 purge going over the catalyst after the reactor had been installed in a constant temperature sand bath. The reactor was at about 3 psig of pressure. HF feed was introduced to the reactor (via the vaporizer and superheater) as a co-feed with the N2 for 15 minutes when the N2 flow was stopped. The HF flow...
example 2
[0051]The fluorinated Cr2O3 catalyst deactivated after 650 hours of on-stream time as described in Example 1 was regenerated by the following procedure.
[0052]The reactor was heated to 300° C. while flowing N2 at the rate of 5000 cc / min. After reactor temperatures were stabilized, synthetic air was introduced. Air flow was started with a rate that gave 0.5% O2. Gradually, with 0.25% O2 increments, air flow was increased to achieve O2 concentration of 2.0%. Then reactor hot-spot was brought to 360° C. And then air flow rate was gradually, with 0.5-1.0% increments, increased to achieve O2 concentration of 5.0%. Careful adjustments of reactor heater temperature were needed to avoid overheating reactor above 380° C.
[0053]The reactor temperature was maintained at a 360-375° C. catalyst bed hot spot temperature while flowing 5% O2 / N2 until the hot spot reached the top of the catalyst bed. Then, without changing reactor heater temperature, O2 flow was maintained until reactor temperature ap...
example 3
[0055]This example illustrates the Step 1 continuous vapor phase fluorination reaction of 1,1,1,2,3-pentachloropropane (HCC-240db)+3HF→2-chloro-3,3,3-trifluoropropene (HCFO-1233xf)+4HCl. The fluorination catalyst for the experiment was fluorinated Cr2O3.
[0056]The same continuous vapor phase fluorination reaction system as described in Example 1 was used for Example 3. The HCC-240db+3HF→HCFO-1233xf+4HCl reaction was run at a 15:1 mole ratio HF to HCC-240db, contact time of 15 seconds, and a reaction temperature of 255° C. GC analysis of the reactor effluent showed 100% conversion of HCC-240db and 98.3% selectivity of HCFO-1233xf on a molar basis. The details of Example 3 are presented in Table 1.
TABLE 1Exp# 71 HCC-240db + 3HF ----> HCFO-1233xf + 4 HClHFO-1234yf / HFC-HCFC-HCFO-* HCC-Component245cb244bb1233xf240dbothersSelectivity0.50.698.3100.00.6Fluorinated Cr2O3 catalyst, 15:1 mole ratio HF to HCC-240db, contact time 15 seconds, Reaction Temperature = 255° C.* Conversion
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com