Coated articles and related methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
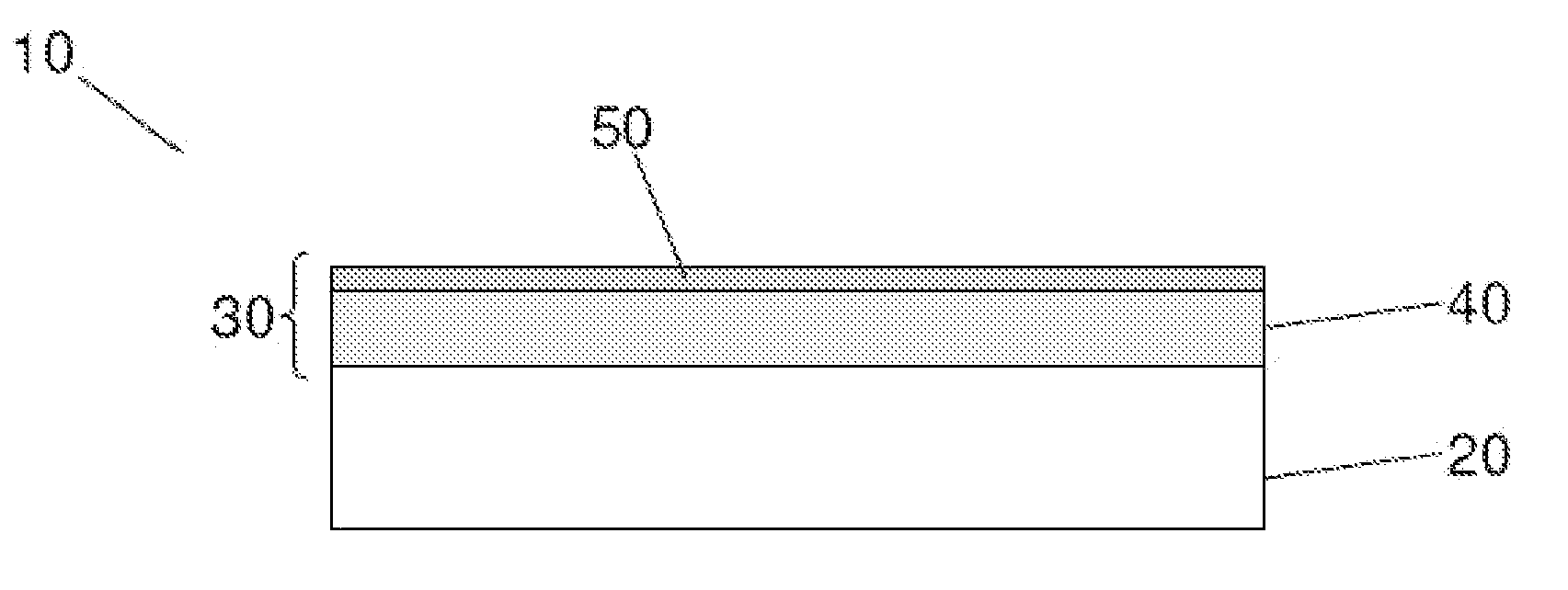
[0063]In the following example, articles coated with Ni—W alloys were produced by aqueous electrodeposition. The article to be coated was immersed in a solution, and a current was applied for electrodeposition. The components of the solution used for deposition are listed in Table I, along with some of the conditions used in the electrodeposition process. The pH of the solution was balanced to a value of 8.0 using ammonium hydroxide. Reverse pulsed current was applied with the characteristics shown in Table I. The reverse pulse scheme used here is similar to that taught by U.S. Patent Publication No. 2006 / 02722949. Several coatings were prepared atop brass substrates, using a counter electrode of stainless steel.
TABLE 1Deposition conditions for experiments one and two.Citrate ions63g / LNickel (from nickel sulfate)6.5g / LTungsten (from sodium tungstate)32.5g / LForward current pulse time (ms)16Negative current pulse time (ms)4Positive current density (A / cm2)0.1Negative current density (A...
example 2
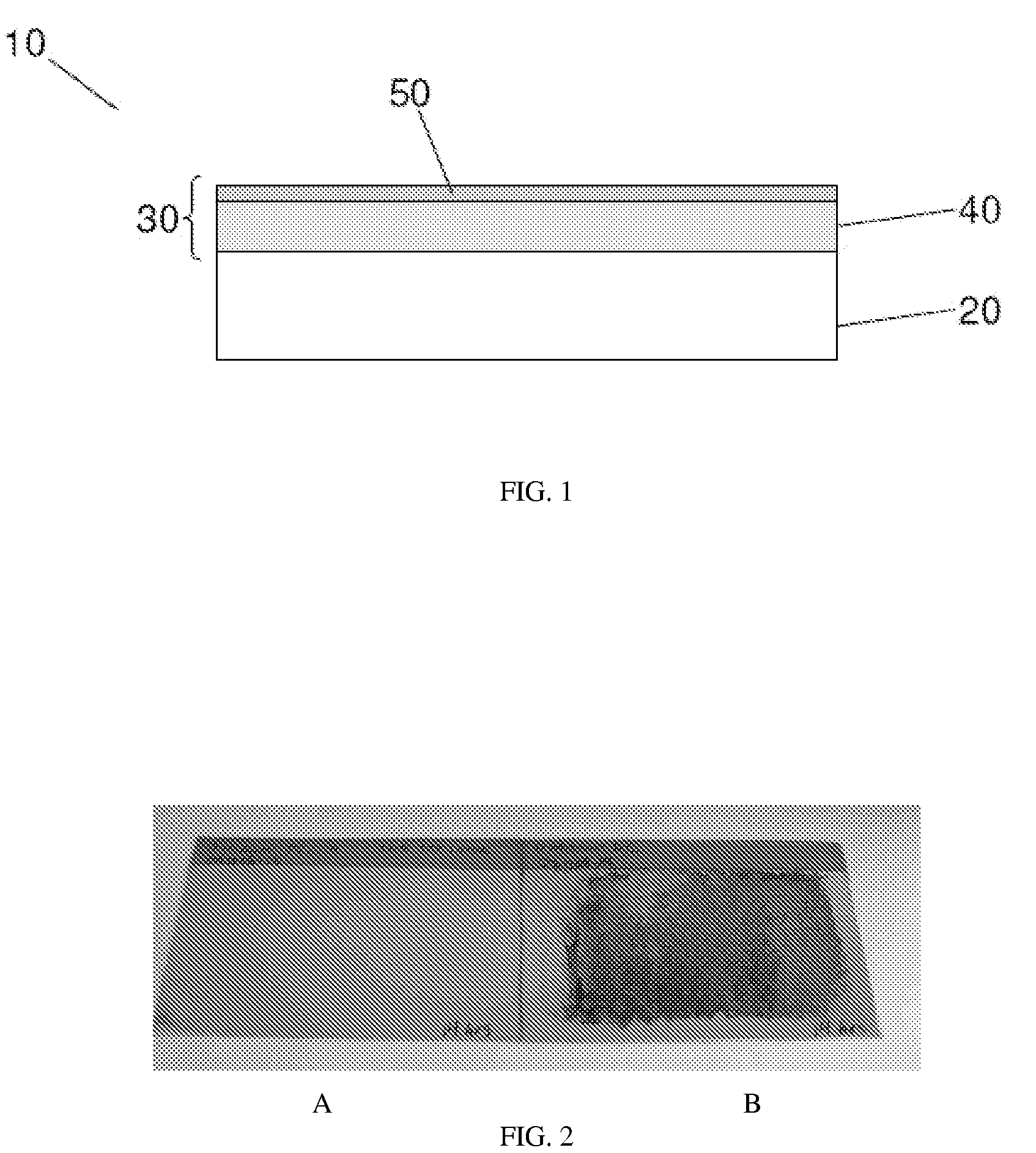
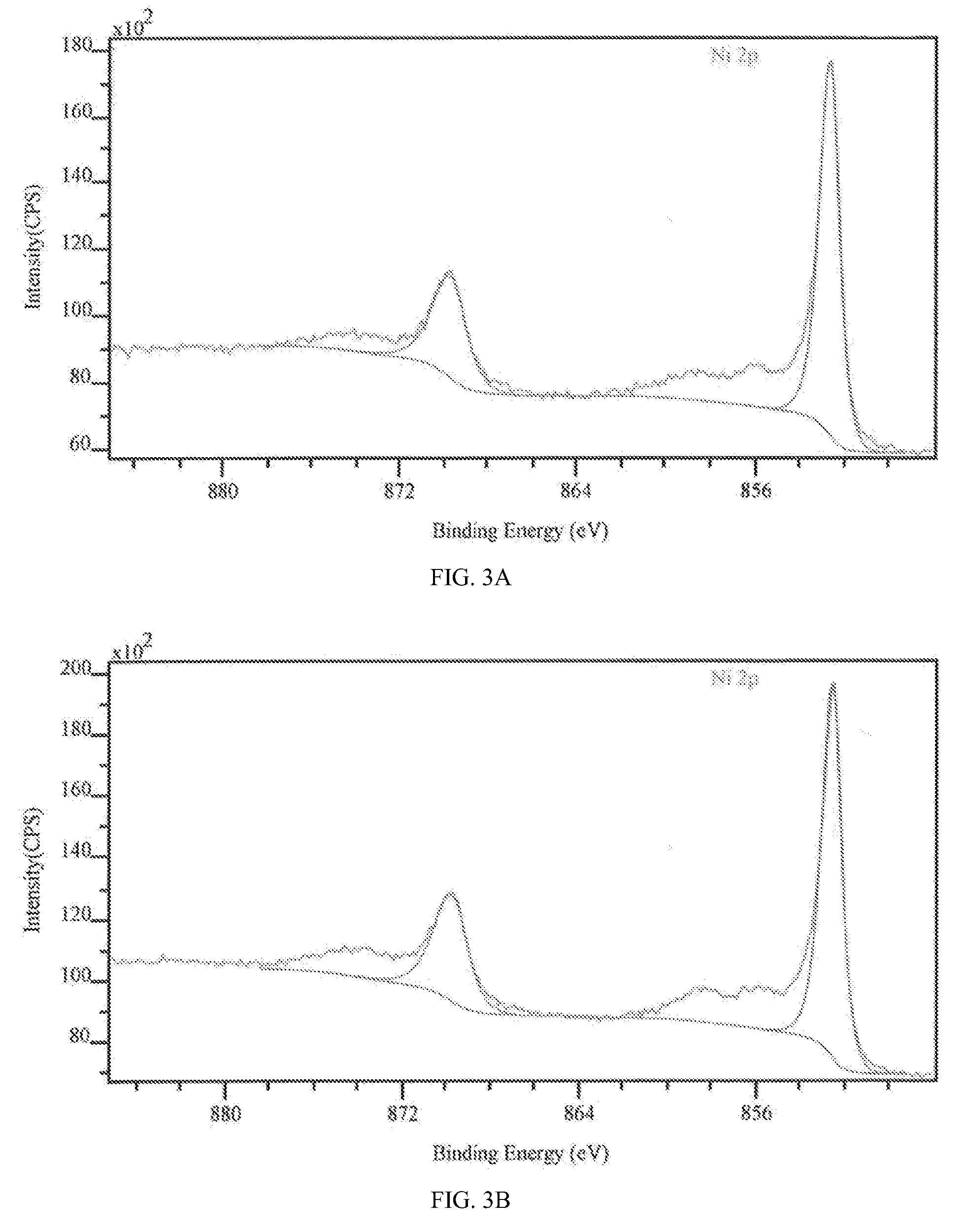
[0070]Various properties of the samples produced in Example 1 were then studied to determine the effect of the character and composition of the outermost surface of the coatings on the corrosion properties of the sample. The compositions of the two specimens, Sample A and Sample B, were measured using Auger electron spectroscopy, prior to exposure to a corrosive environment. The Auger electron spectroscopy results indicated that the near surface regions of the two coatings were different.
[0071]Sample A had a surface composition comprising Ni (˜62 at %), W (˜22 at %) and O (˜16 at %). When the oxygen was excluded from the analysis, the ratio of the metals is about 75 at % Ni: 25 at % W, or, expressed in weight percentages, 49 wt % Ni: 51 wt % W. This composition was reasonably close to the bulk measurement provided by the XRF results, with a slight difference likely due to the presence of oxide on the surface. Sample B had a very different surface composition, comprising Ni (˜86 at %...
example 3
[0077]A variety of additional coating samples were produced and their properties studied. For example, Sample C and Sample D were produced using the methods described in Example 1 to produce Samples A and B, respectively, except that organic additives (i.e., leveling agents, wetting agents, brightening agents) were added to the electrodeposition bath in the amount of less than 1 g / L. Those of ordinary skill in the art would recognize that levelers, brighteners, ductility agents, wetters and the like may be commonly used in such small quantities in electrodeposition baths, and that many combinations of these may be present in different baths. In this Example, the presence of small concentrations of organic additive did not change the major results reported above for the Samples A and B. Sample C exhibited CASS corrosion in only a few hours, with quite substantial corrosion after 4 hours. Sample D, however, comprised the corrosion resistant top portion and had a CASS corrosion lifetim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com