Electrodes with Multilayer Membranes and Methods of Using and Making the Electrodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
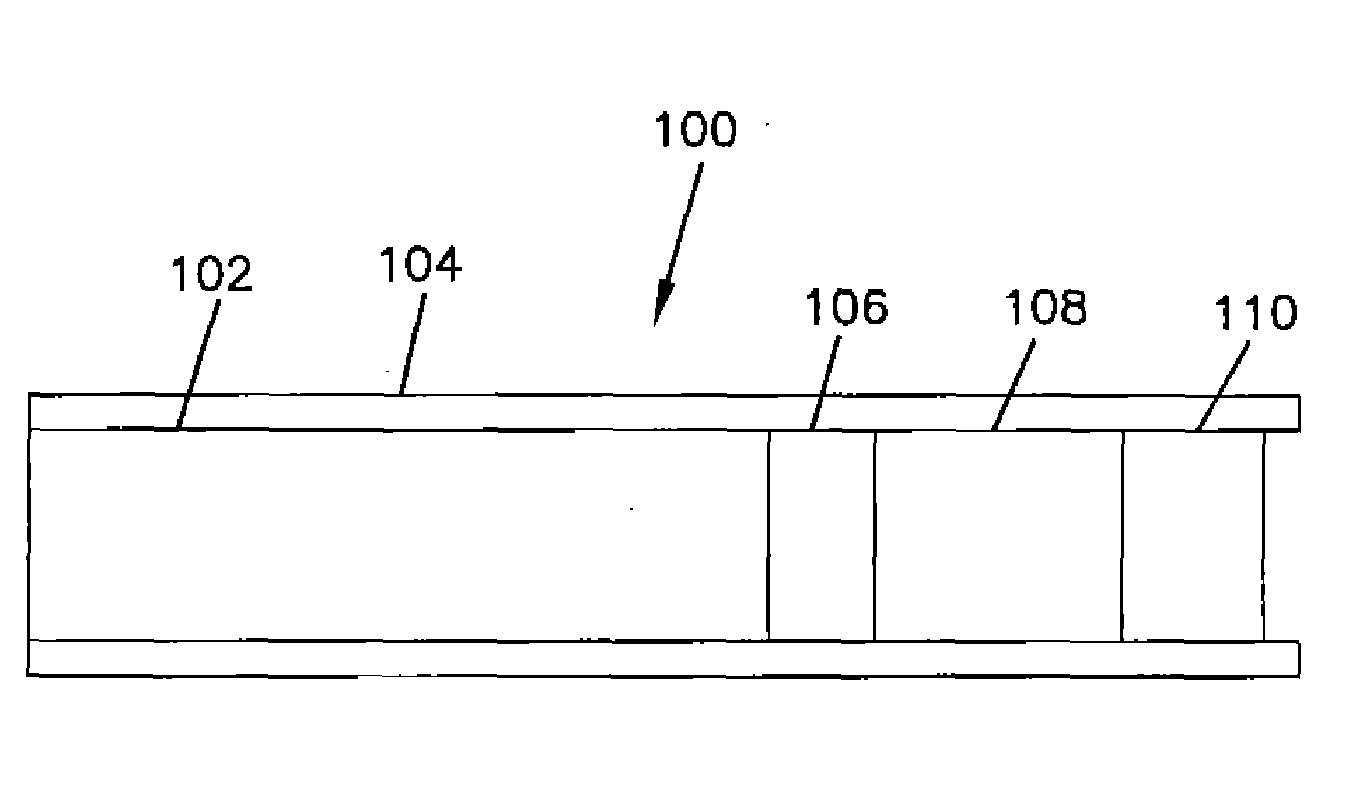
[0041]Formation of Glucose Electrodes. Miniature gold electrodes were structurally similar to those described in Csöregi et al., Anal. Chem. 66, 3131 (1994) and U.S. Pat. No. 5,593,852, both of which are incorporated herein by reference. The electrodes were made of polyimide-insulated 0.25 mm gold wire, which was cut to 5 cm long pieces. At one end, the insulation was stripped from 0.5 cm of the wire to make the electrical contact. At the other end, a 90 μm deep polyimide-walled recess was formed by electrochemically etching away the gold under galvanostatic control by an EG&G PARC 273A potentiostat / galvanostat.
[0042]The tip of the gold wire at the bottom of the shielded recess was coated with the transduction (sensing) layer; a micro-membrane; and a biocompatible layer. The first and third layers were formed by micropipetting polymer solutions onto the gold surface under a microscope, using a micromanipulator. The micro-membrane was formed by dip and rinse cycles.
[0043]The sensing ...
example 2
[0046]In Vitro Experiments using the Electrode of Example 1. In vitro experiments were carried out in a stirred, water-jacketed electrochemical cell in 0.15 M NaCl, 0.02 M phosphate buffer solution with pH 7.1. The cell had a saturated Ag / AgCl reference electrode, a platinum counter electrode and the modified 0.25 mm gold wire tip working electrode, as described in Example 1. Unless otherwise stated the working electrode was poised at 400 mV vs. Ag / AgCl, and the cell was maintained at 37° C. with an isothermal circulator (Fisher Scientific, Pittsburgh, Pa.). The potential was controlled by a CHI832 electrochemical detector (CH Instrument, Austin, Tex.) and a PC collected the data.
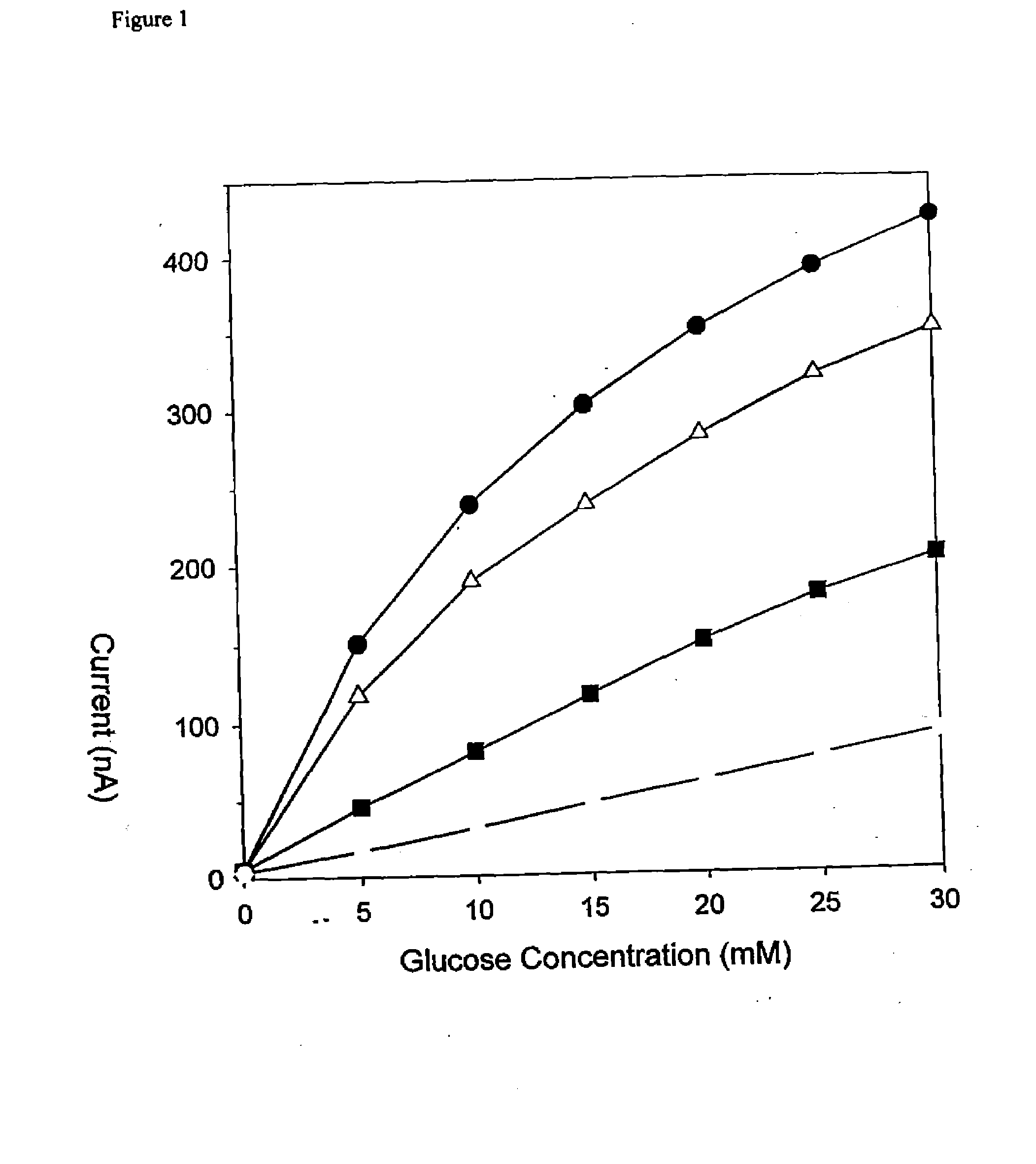
[0047]FIG. 1 illustrates the dependence of the sensitivity on the number of PAc / PAm / PAc / PAm / PAc / PVPEA sextets where the dashed line indicates no micro-membrane, the squares indicate one sextet, the triangles indicate two sextets, and the circles indicate three sextets. This demonstrates the expansion of the...
example 3
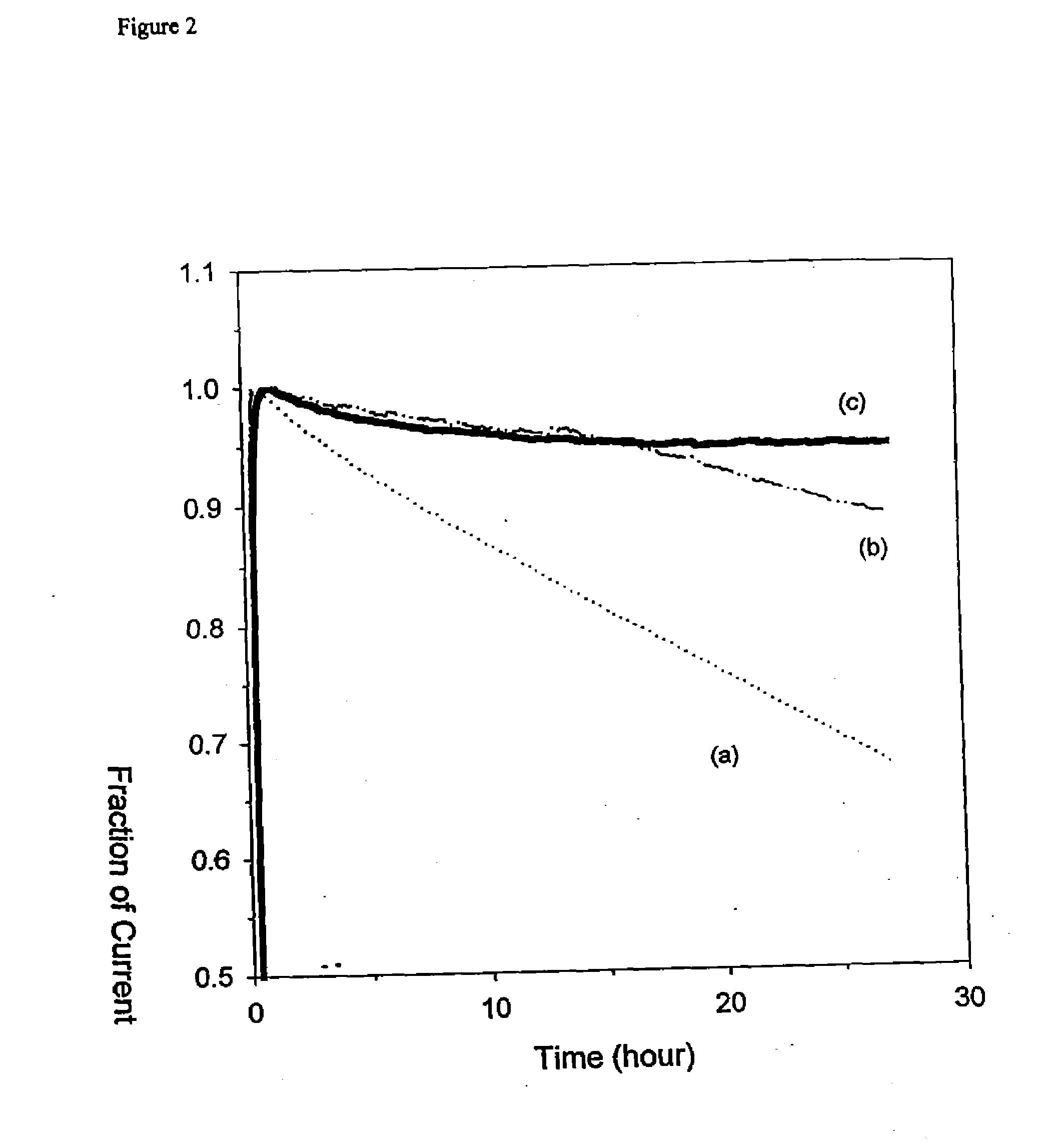
[0053]In Vivo Experiments using the Electrode of Example 1. Male Sprague-Dawley rats, 400-500 g, were pre-anesthetized with halothane (Halocarbon Laboratories, North Augusta, S.C.) and anesthetized by intraperitoneal injection (0.5 mL) of a solution made of equal volumes of acepromazine maleate (10 mg / mL), ketamine (100 mg / mL), and xylazine (20 mg / mL). The animals were shaved about their necks, abdomens, and between their scapulae, and then secured on a homeothermic blanket system (Harvard Apparatus, South Natick, Mass.). A 0.0375-in.-diameter medical grade silicone tube was inserted into the proximal portion of their right external jugular vein and secured with 4-0 silk sutures. A dose of 100 units / kg body weight of heparin solution was then administered, followed by an equal volume of saline to clear the line. A glucose sensor was implanted subcutaneously between the scapulae, using a 22-gauge Per-Q-Cath introducer (Gesco International, San Antonio, Tex.). The sensor was taped to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Area | aaaaa | aaaaa |
| Area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com