Hydrogen-permeable alloy, and hydrogen-permeable film and its production method
a technology of hydrogen-permeable alloys and production methods, applied in metal-working apparatus, electrical equipment,foundry moulds, etc., can solve the problems of brittle intermetallic compounds, brittle alloy bodies, and inability to meet all future fuel cell demand, and achieve the effect of reducing the oxygen content of alloys
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
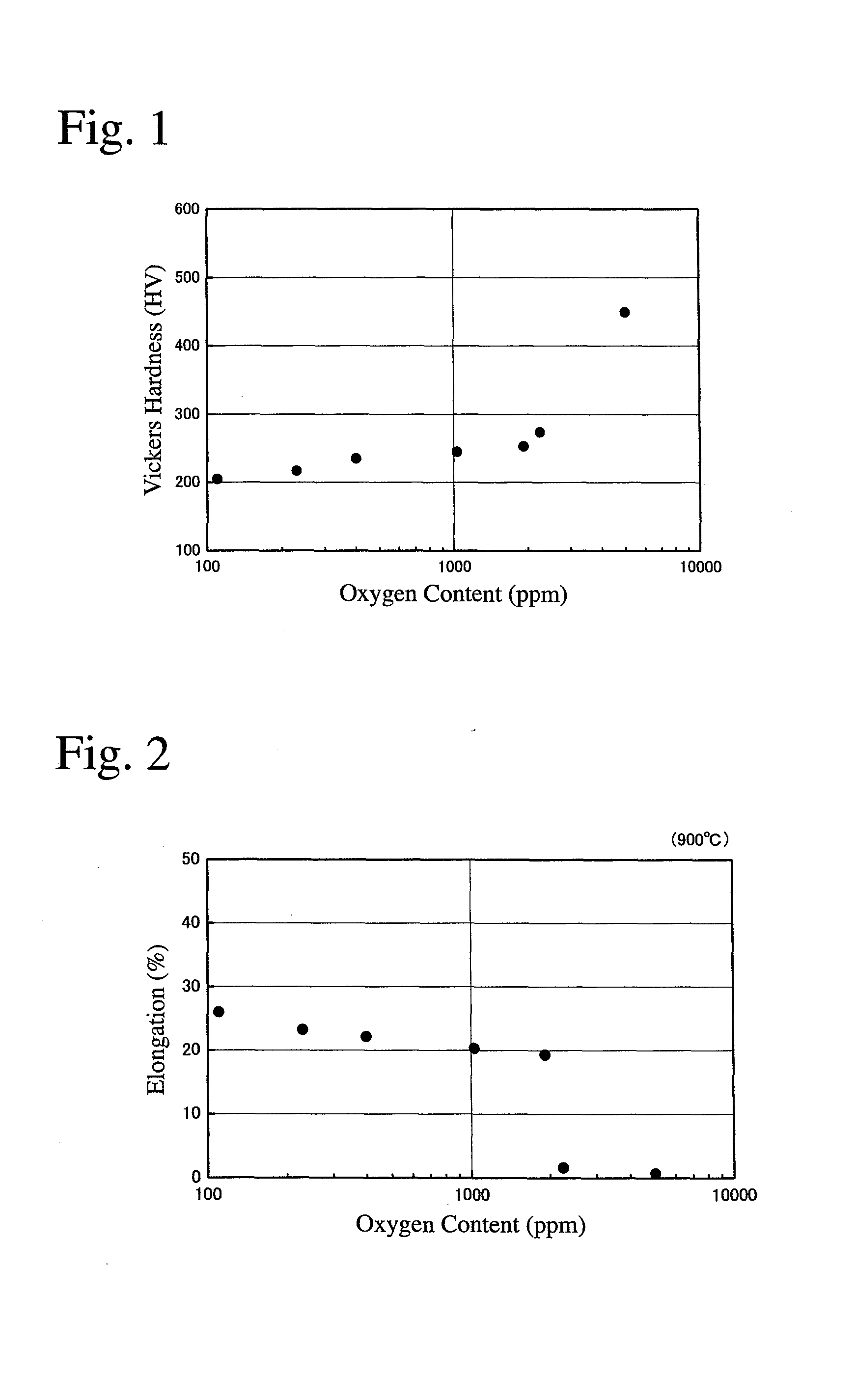
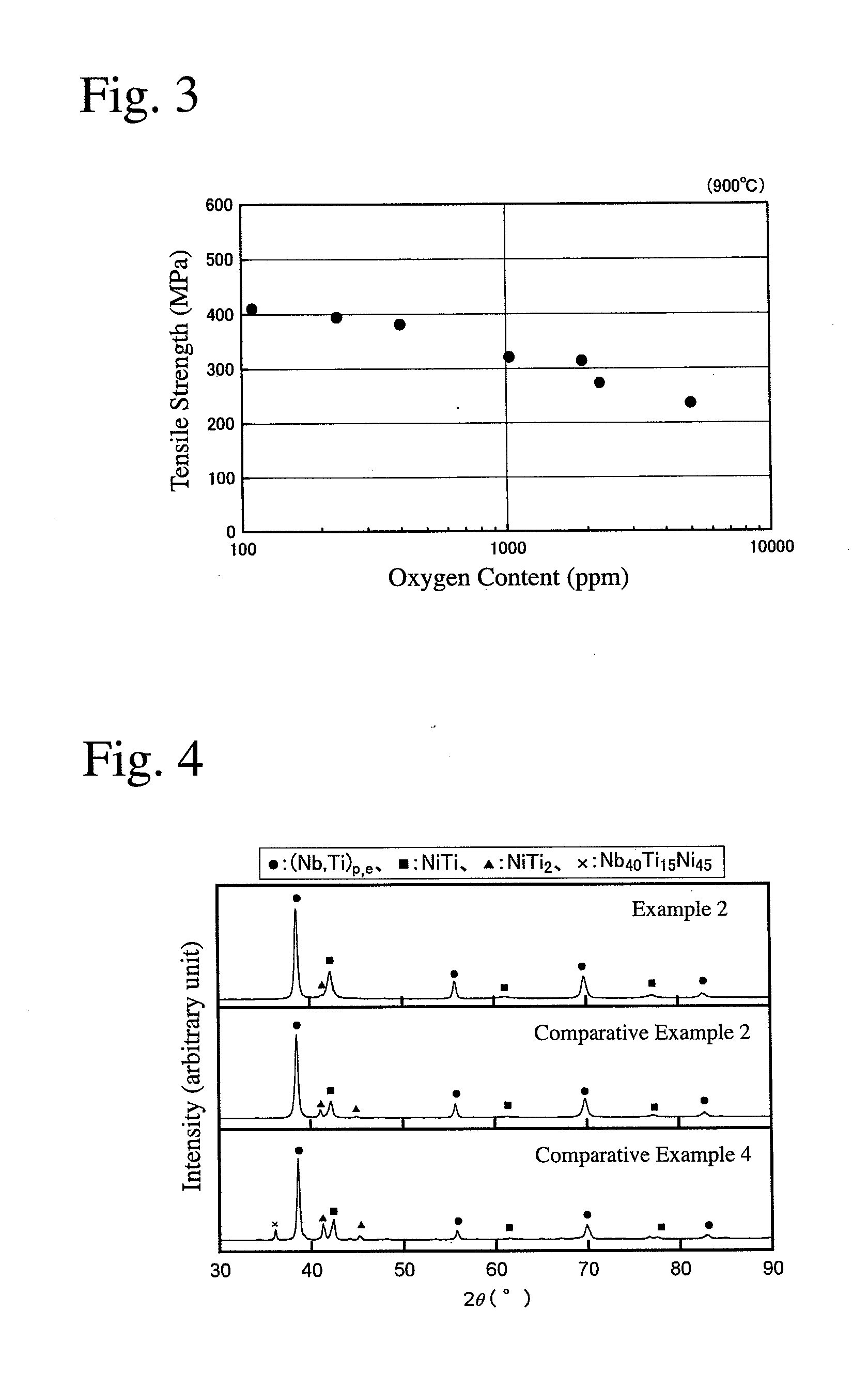
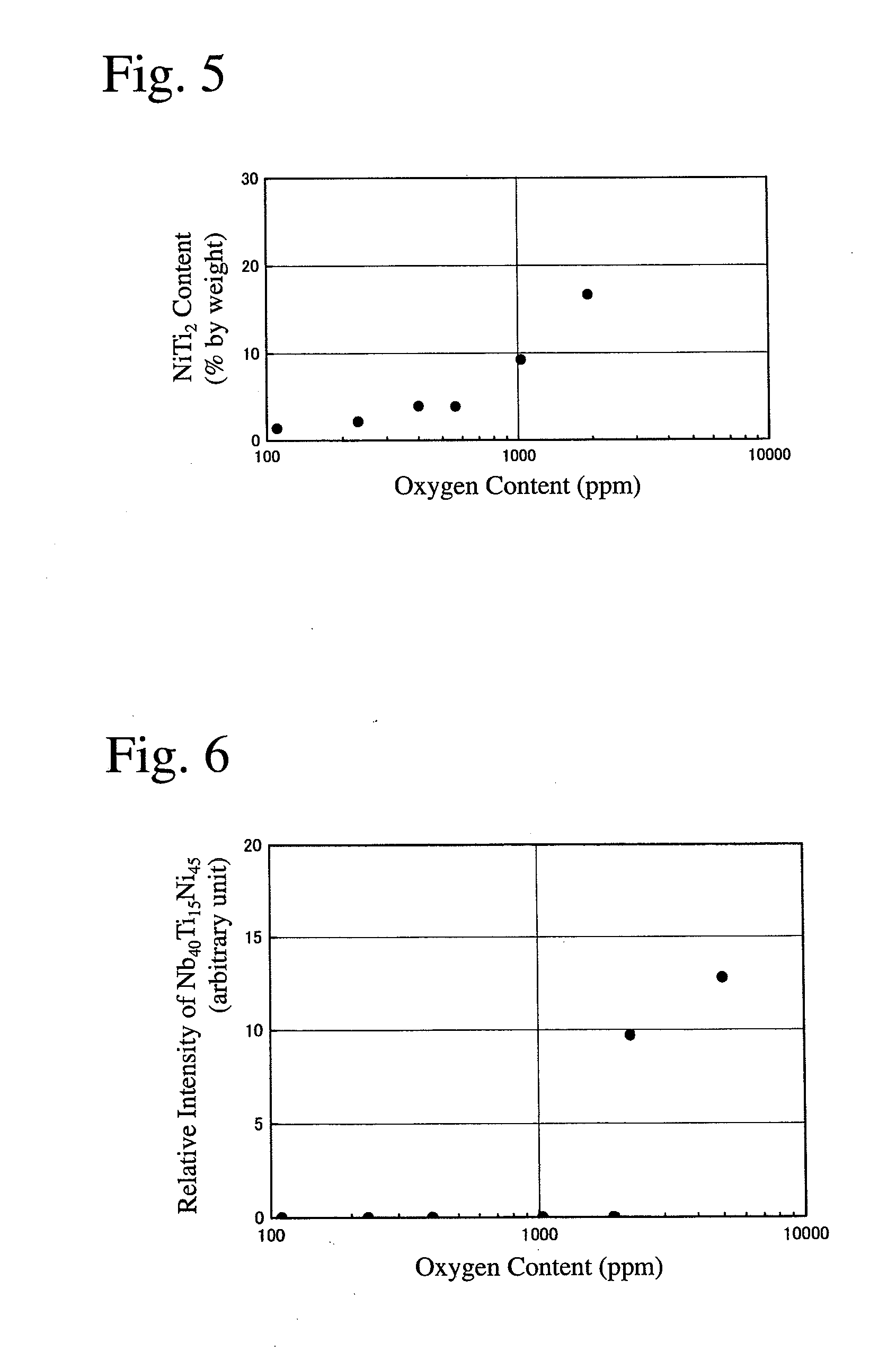
[0045]As alloy materials, pure Nb metal (oxygen content: 10 ppm), pure Ti metal (oxygen content: 140 ppm) and pure Ni metal (oxygen content: 40 ppm) were mixed to a composition of Ni30Nb40Ti30 (atomic %), and metal Ca as a deoxidizer was added to the mixture in an amount of 200 ppm based on the alloy materials. The resultant mixture was charged into a first water-cooled copper crucible in a vacuum arc-melting apparatus. Metal Ti as a getter material for removing oxygen from an atmosphere was charged into a second water-cooled copper crucible in the vacuum arc-melting apparatus. The amount of the getter material was 70% by mass based on the alloy materials.
[0046]After reducing the pressure of the atmosphere in the vacuum arc-melting apparatus to 4.0×10−3 Pa, an Ar gas was introduced, and evacuation was conducted again to 4.0×10−3 Pa. Thereafter, an Ar gas (purity: 99.99%) at 40 kPa was introduced into the apparatus. The getter material was arc-melted to absorb an oxygen gas in the at...
example 2
[0047]A cast Nb—Ti—Ni alloy body was produced from Nb metal (oxygen content: 20 ppm), Ti metal (oxygen content: 250 ppm) and Ni metal (oxygen content: 40 ppm) as alloy materials, in the same manner as in Example 1 except for changing the amount of the getter material to 60% by mass, and the vacuum degree in the vacuum arc-melting apparatus to 5.0×10−3 Pa.
example 3
[0048]A cast Nb—Ti—Ni alloy body was produced from Nb metal (oxygen content: 40 ppm), Ti metal (oxygen content: 250 ppm) and Ni metal (oxygen content: 60 ppm) as alloy materials, in the same manner as in Example 1 except for changing the amount of the getter material to 50% by mass, and the vacuum degree in the vacuum arc-melting apparatus to 5.0×10−3 Pa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Vickers hardness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com