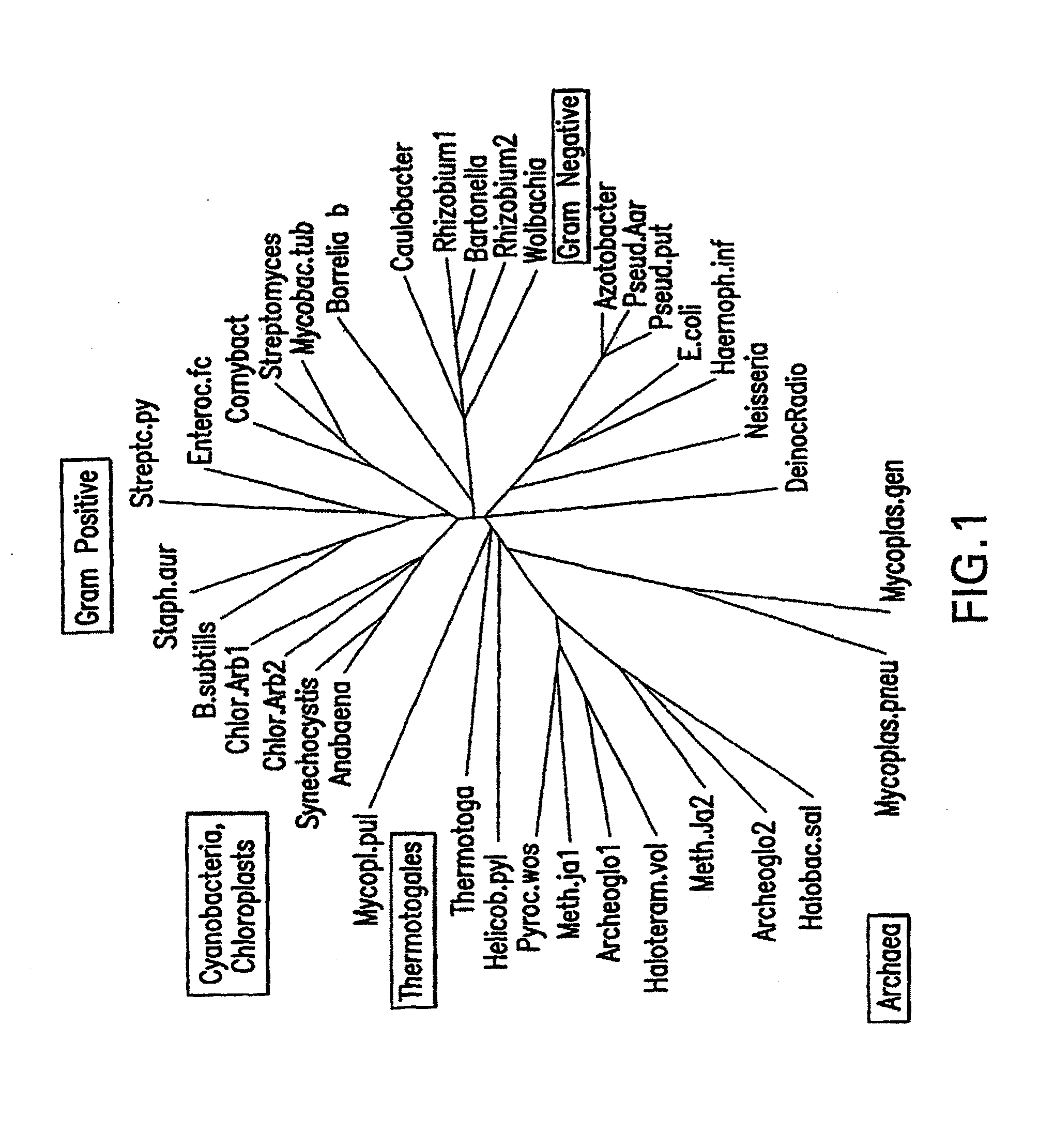
Inhibitors of ftsz and uses thereof
a technology of inhibitors and antibacterial agents, applied in the field of antibacterial agents, can solve the problems of inability to understand the mechanism of action, the development of tolerance and/or resistance to antibacterial agents is a significant threat to the ability to treat disease, and the need for continual development of new agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Anti-Tubercular Assay
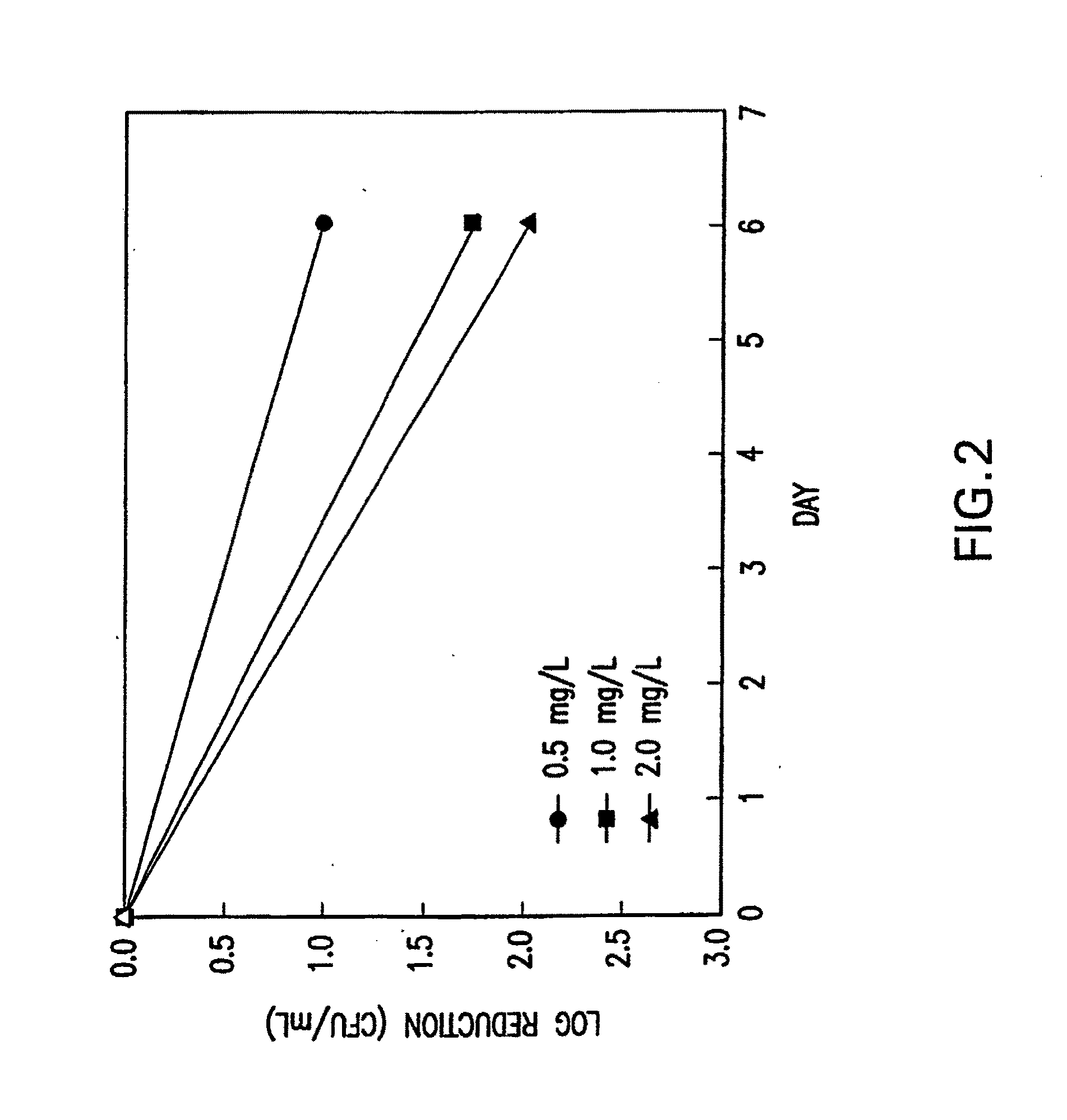
[0162]Most anti-tubercular assays were conducted by the NIH Tuberculosis Anti-bacterial Acquisition and Coordination Facility (TAACF) screening facility against M. tuberculosis H37Rv (ATCC 27294; American Type Culture Collection, Manassas, Va.) using a BACTEC 460 radiometric system to determine the minimum inhibitory concentration (MIC99). Rifampin was the positive control. The MIC99 was also determined for SRI-7614 and SRI-3072 against strains of M. tuberculosis resistant to isoniazid, rifampin, ethambutol, kanamycin, pyrazinamide, thiacetazone, or cycloserine (Table 5). Concurrent with the determination of MIC99, compounds were tested for cytotoxicity (IC50) in Vero cells. After 72 h exposure, viability was assessed on the basis of cellular conversion of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS) into a formazan product using the Promega CellTiter 96® AQueous Non-radioactive Cell Proliferation assay. The...
example 2
Purification and Characterization of FtsZ from M. tuberculosis
[0165]The purification of FtsZ was performed as described previously (White et al. J. Bact. 182:4028-4034, 2000). The M. tuberculosis FtsZ coding sequence was sub-cloned into the NcoI site of pET15b (Novagen). This plasmid, pJD168, was used to transform E. coli BL21 (DE3) / pLysS. Cells were incubated at 32° C. in LB media containing 0.4% glucose for 1 h. Five hundred μl of transformed cells were added to 250 ml of fresh LB media containing 0.4% glucose, 100 μg / ml ampicillin, and 34 μg / ml chloramphenicol and incubated overnight at 32° C. The cells were pelleted by centrifugation at room temperature. The cells were resuspended in four liters of prewarmed LB media containing 0.2% glucose, 100 μg / ml ampicillin, and 34 μg / ml chloramphenicol; the culture was shaken with good aeration at 32° C. When the culture reached an A600 of ˜0.4, expression of FtsZ was induced with 1 mM isopropyl β-D-thiogalactoside. Cells were harvested 3...
example 3
Light Scattering Assay for FtsZ Polymerization
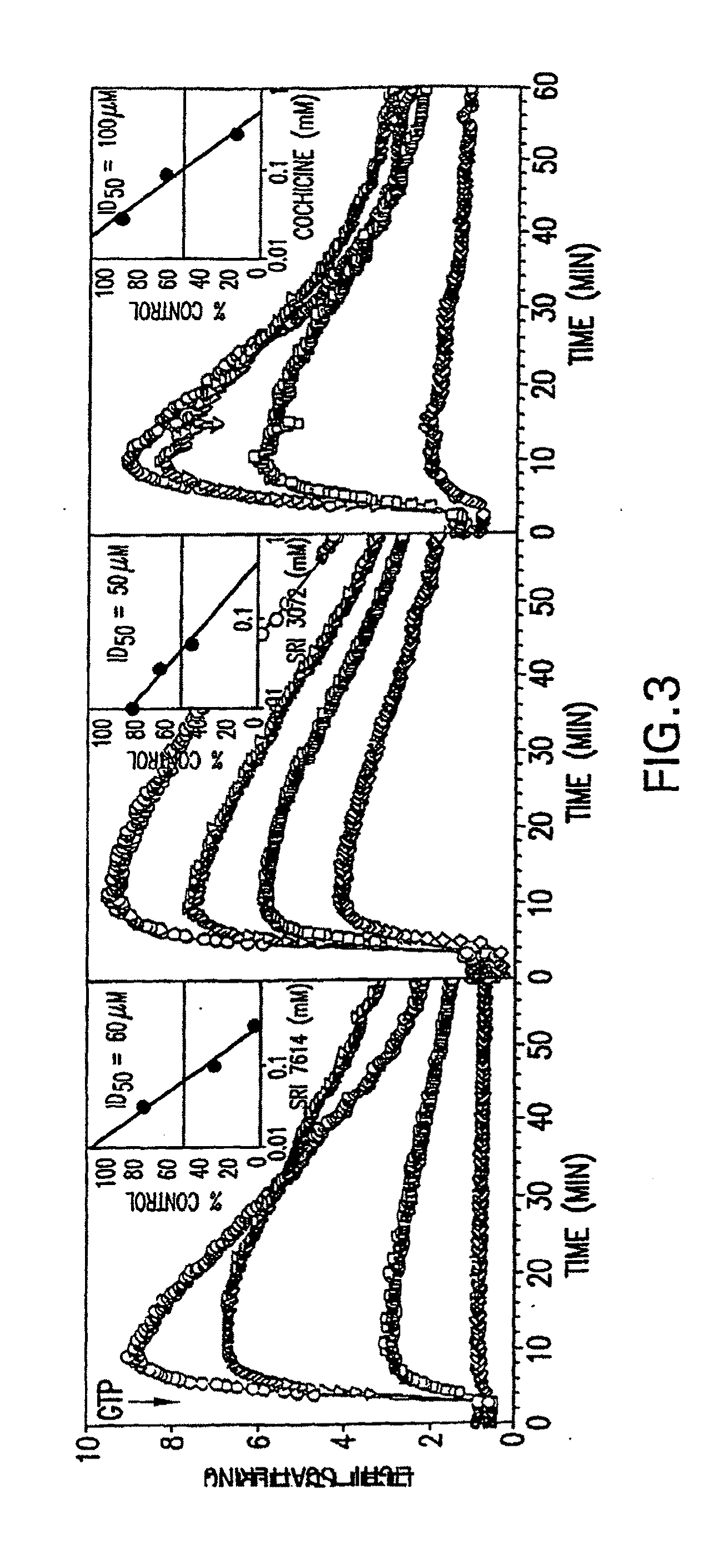
[0170]The polymerization and depolymerization of purified FtsZ was followed by the method described by Mukherjee and Lutkenhaus for E. coli FtsZ (J. Bact. 181:823-832, 1999). Light scattering was measured in a thermostatically (30° C.) controlled Aminco-Bowman series 2 luminescence spectrometer using 0.5 ml quartz cuvets (cell 2×10 mm, Hellma). Excitation and emission wavelengths were 400 nm with a slit width of 2 nm. The gain was typically set at 540 V but was increased if needed to give a maximum response around 8. FtsZ (500 μg / ml; 13 μM) was incubated in 50 mM MES-NaOH pH 6.5, 100 mM KCl, and 5 mM MgCl2 to establish a baseline. GTP (40 μM) was added (final volume 300 μl) and the increase in light scattering measured for an additional 50-60 min. Changes in concentrations of a component for a particular experiment are indicated in the text or figure legend.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com