Systems and methods for applying an antimicrobial coating to a medical device
a medical device and antimicrobial coating technology, applied in the field of systems and methods for using antimicrobial coatings, can solve the problems of failure to flush the device regularly, affecting the health of patients, and affecting the quality of life of patients, so as to improve the antimicrobial coating and reduce the complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
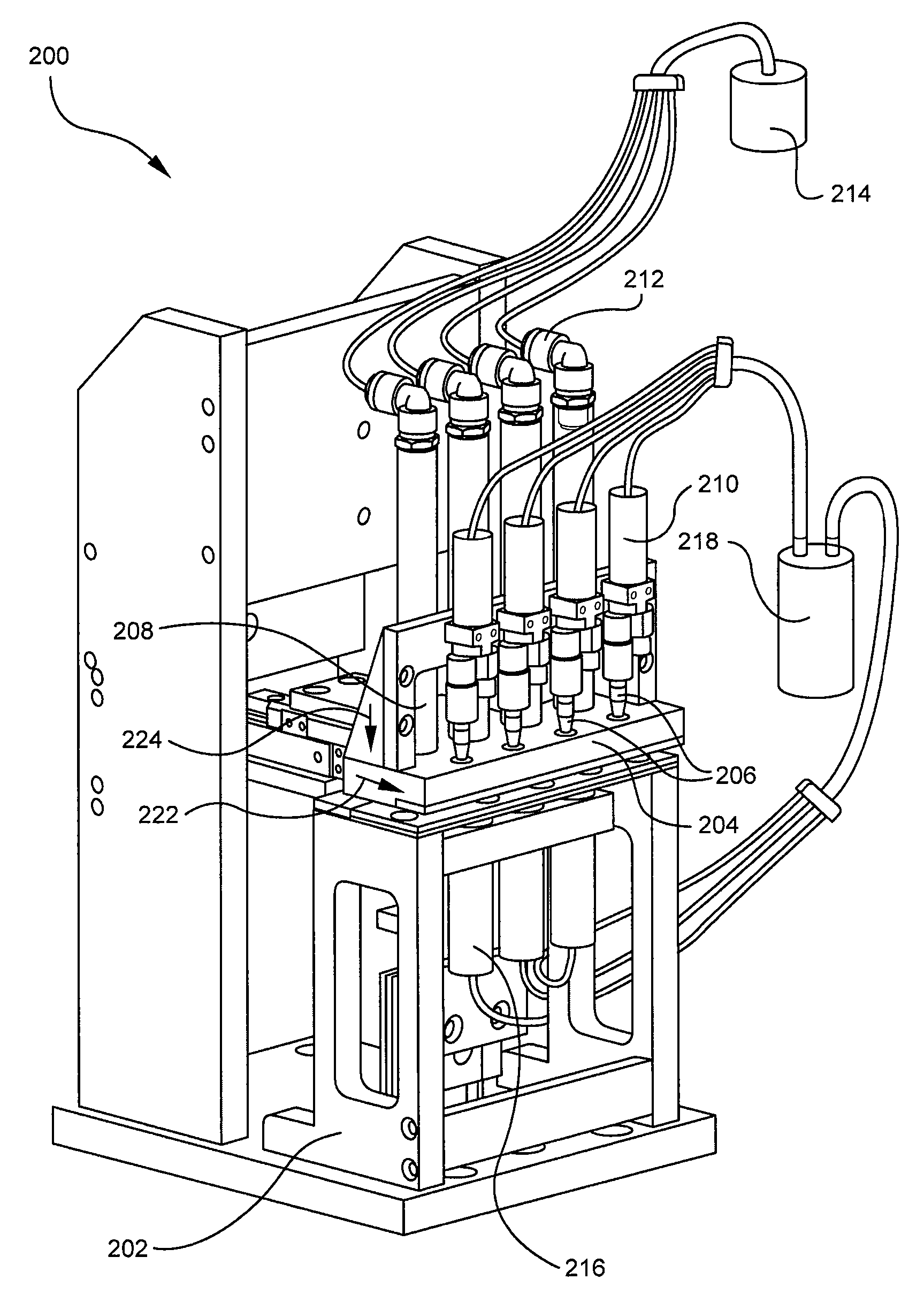
[0031]The described invention relates to methods and compositions for coating one or more surfaces of a medical device with an antimicrobial coating. Once the antimicrobial coating is cured onto the medical device, an antimicrobial agent in the coating can gradually diffuse out of the coating when the coating is softened by IV fluids or other types of fluids. Accordingly, microbes that come into contact with the coated surface of the medical device can be killed and the medical device may remain sanitary for a prolonged period of time.
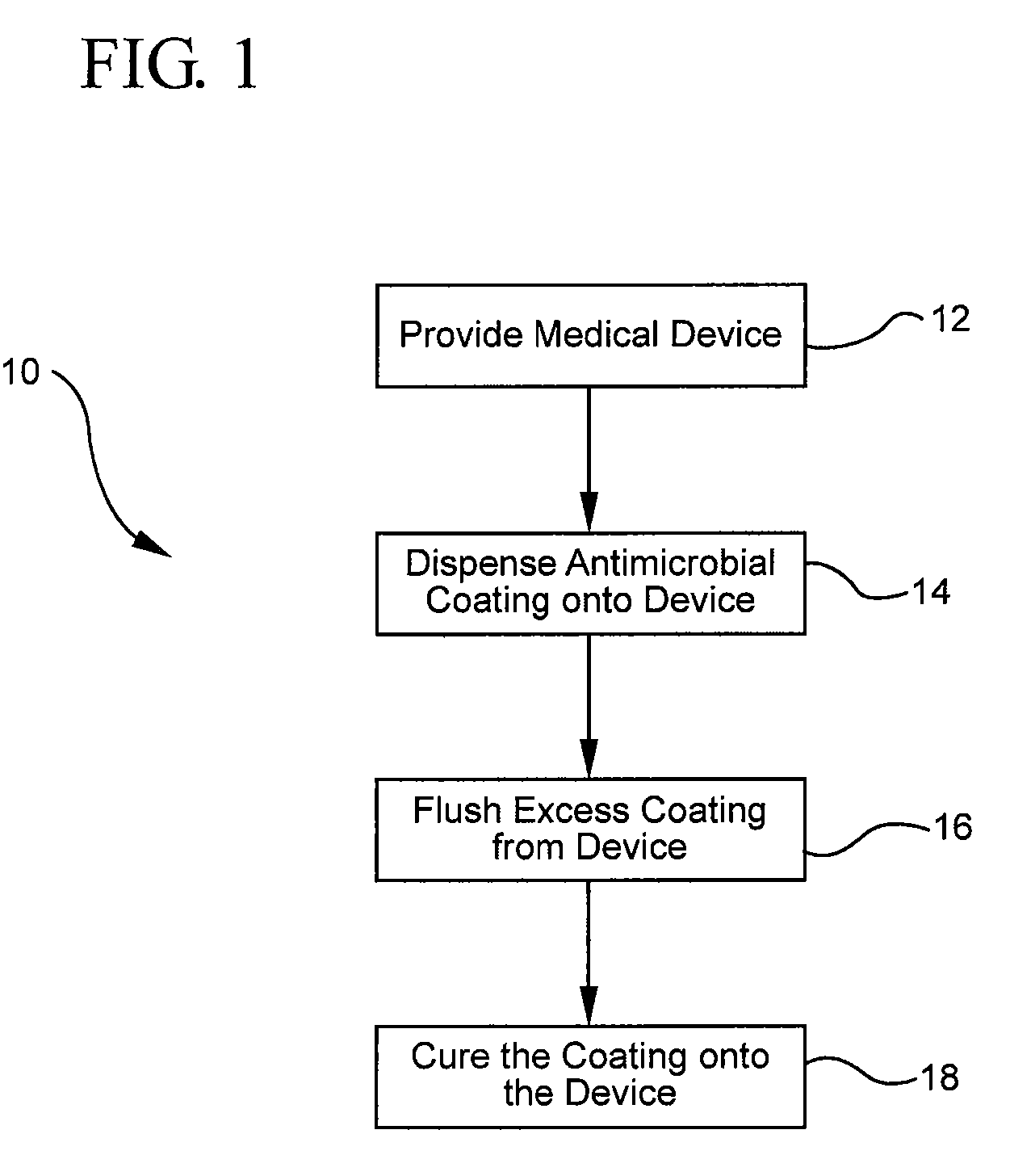
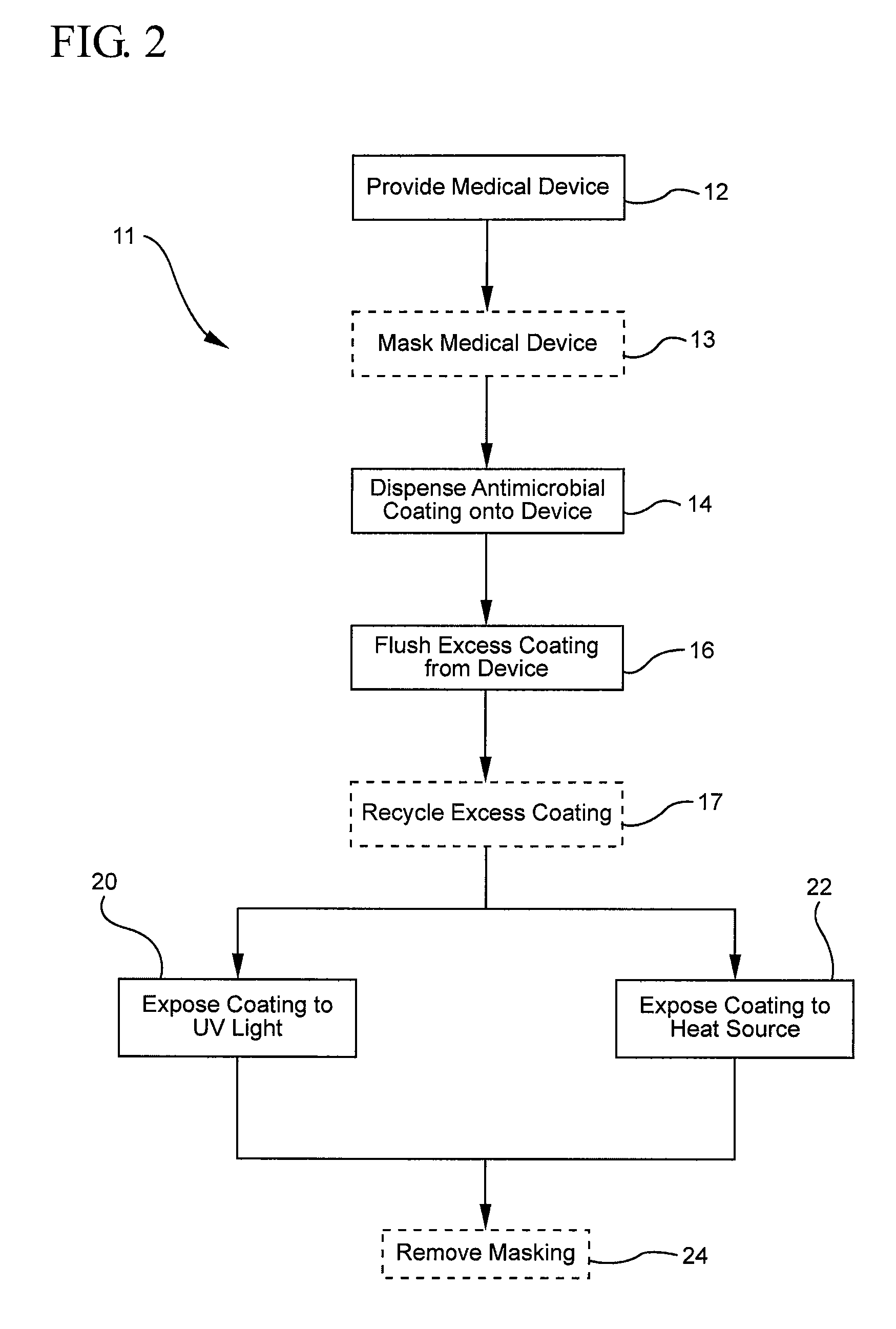
[0032]FIG. 1 illustrates a representative embodiment of the described coating methods. Specifically, FIG. 1 shows that the method 10 for coating a medical device with an antimicrobial coating generally comprises providing a medical device 12, dispensing an antimicrobial coating onto the device 14, flushing excess coating from the device 16, and curing the coating to the device. In order to provide a better understanding of the described coating method,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| period of time | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| air pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com