Rheumatoid arthritis-preventive agent for oral intake
a technology of rheumatoid arthritis and oral intake, which is applied in the direction of drug compositions, peptide/protein ingredients, peptide sources, etc., can solve the problems of prescription for such drugs, and achieve the effect of inhibiting arthritis and excellent safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
[0052]1 g of casein derived from cow's milk (manufactured by NIPPON NZMP (Japan) LTD.) was added to 99 g of distilled water adjusted to about 80° C., and the resulting mixture was thoroughly stirred. The pH of the mixture was adjusted to 7.0 with 1N sodium hydroxide solution (manufactured by WAKO PURE CHEMICAL INDUSTRIES, LTD.), and the temperature was adjusted to 20° C., to prepare a substrate solution.
[0053]To this substrate solution, a commercially available enzyme (Sumizyme FP, registered trademark, manufactured by SHIN NIHON CHEMICAL CO., LTD.), which is derived from Aspergillus oryzae and contains at least metal protease, serine protease, neutral protease I, neutral protease II, and leucine amino peptidase, was added at the enzyme / casein ratio of 1 / 25 by weight, and the resulting mixture was reacted at 50° C. for 14 hours. Then the reaction product was autoclaved at 110° C. for 10 minutes to inactivate the enzyme, thereby obtaining a solution of enzymatic hydrolysate of casein...
production example 2
[0064]Lactobacillus helveticus CM4 strain (deposited at International Patent Organism Depositary, National
[0065]Institute of Advanced Industrial Science and Technology, Tsukuba Central 6, 1-1-1 Higashi, Tsukuba-shi, Ibaraki, Japan, under accession number FERM BP-6060 on Aug. 15, 1997) (referred to as CM4 strain hereinbelow) was provided. The CM4 strain has been deposited under the above-mentioned accession number under the Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure, and has already been patented.
[0066]A commercially available skim milk was dissolved in distilled water at 9% (w / w) solid content, subjected to high temperature pasteurization in an autoclave at 105° C. for 10 minutes, and cooled to the room temperature. Then the solution was inoculated with 3% (v / w) of a fermentation liquid of CM4 strain starter (cell count 5×108 cells / ml), and fermented under static conditions at 37° C. for 24 hours to obtain a...
example 2
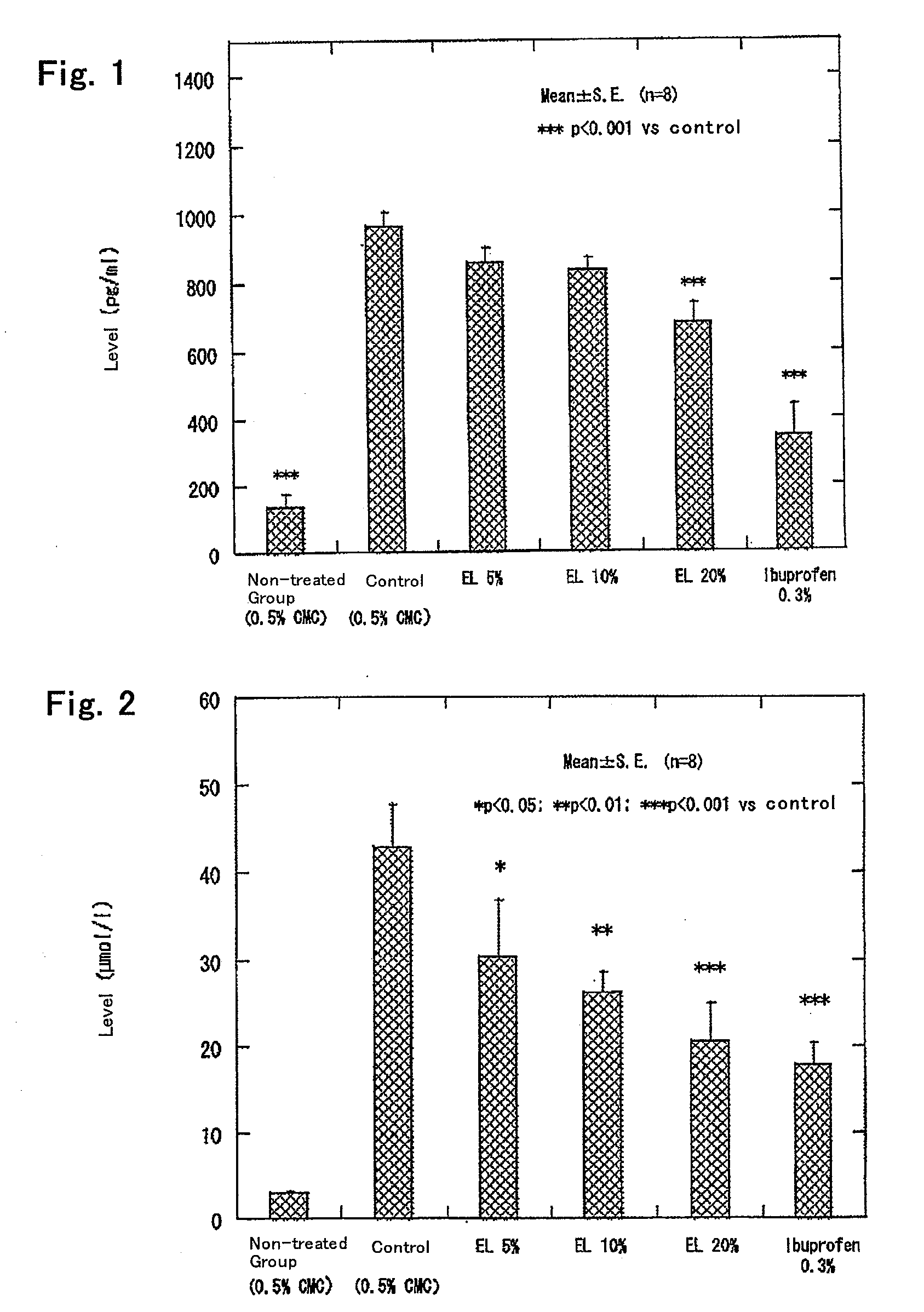
[0076]The casein enzymatic hydrolysate prepared in Production Example 1 (Example 2) as a test material and Ibuprofen (manufactured by WAKO PURE CHEMICAL INDUSTRIES, LTD.) as a positive control were provided.
[0077]As in Example 1, the adjuvant for sensitization was prepared on the day of sensitization with the adjuvant as will be discussed later.
[0078]Female Lewis rats (SPF) of 8 weeks of age were preliminarily bred for 7 days before subjected to the test. Throughout the preliminary breeding period and the test period, the rats were raised in SPF barrier facilities at a room temperature of 24±3° C. and a relative humidity of 55±15%, with the lighting hours of 7:00 to 19:00 and the air exchange rate of 18 times / hour. The rats were housed 2 to 3 per cage, and all the groups were allowed free access to solid feed (trade name MF, manufactured by ORIENTAL YEAST CO., LTD.) and sterilized deionized water. The rats were identified by picric acid applied on the fur.
[0079]After the preliminary...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average chain length | aaaaa | aaaaa |
| swelling | aaaaa | aaaaa |
| RA inhibitory effect | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com