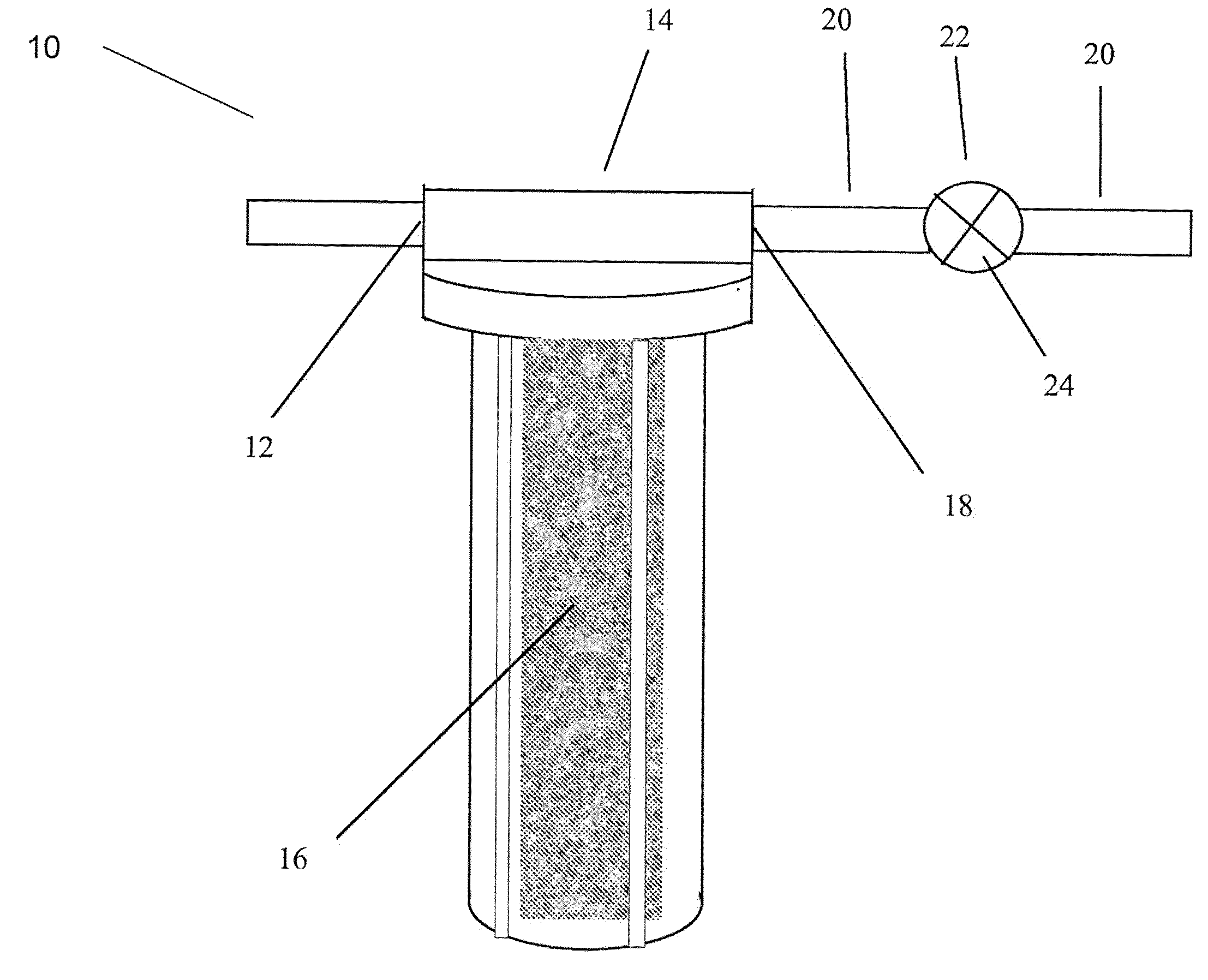
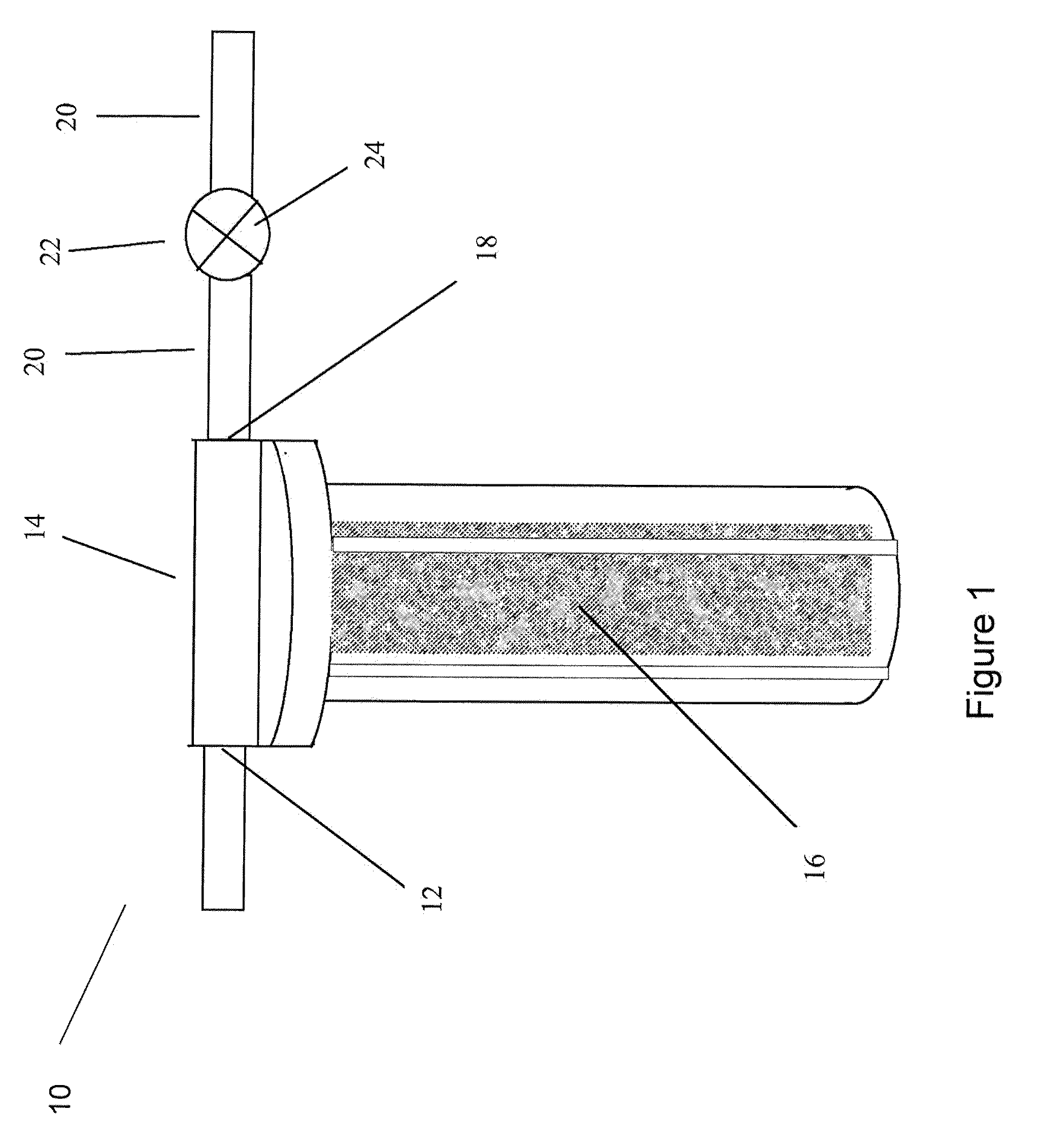
Methods and apparatus for controlling water hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0106]Three 1 pound resin samples were prepared by loading them with H+, Ca++, and Mg++. The magnesium loaded sample was prepared according to the following procedure. A weak acid cation resin, Lewatit S 8528 obtained from the Lanxess Company, was soaked is 500 grams of NaOH beads and 2500 ml of softened water for 24 hours. The pH was approximately 12-13. After soaking, the resin was then rinsed thoroughly with softened water three times until the pH of the rinse water was below 11. The resin was soaked in 2500 ml of softened water with 700 grams of a MgCl2.6H20 composition for 4 days.
[0107]The resin was thoroughly rinsed with softened water three times. The final pH of the rinse water was approximately 7.5-8.5.
[0108]To load the resin with Ca++, the same procedure was used as described above for the MG++ resin, only the resin was soaked with CaCl2 composition. The H+ form of the resin, was the resin itself, without any cations loaded onto it.
[0109]Water was then treated with each of...
example 2
[0112]Water with 17 grains of water hardness was treated with two pounds of Watts OneFlow media, commercially available from Watts, at a rate of about 5 gallons per minute. In addition, water with 17 grains of water hardness was treated with a magnesium loaded weak acid resin according to the present invention at the same conditions. An alkalinity source including 800 ppm of sodium carbonate was added to each of these water samples, as well as to a control sample of untreated water. The results are shown in FIG. 3. As can be seen from this Figure, both the control and the Watts treated water had a signification precipitation of water hardness. The water treated according to the present invention (shown as the right most beaker) showed no signs of a precipitate.
example 3
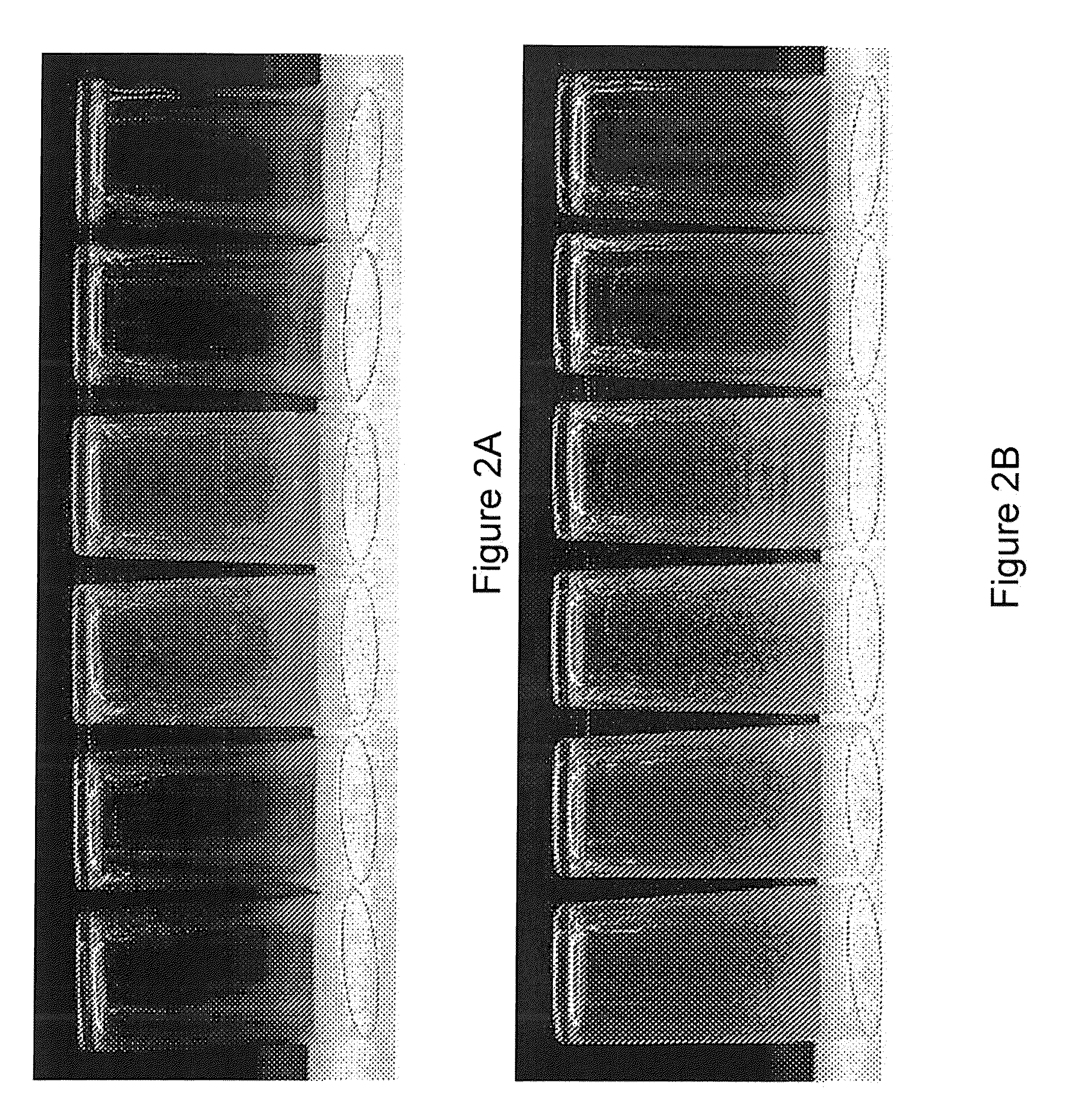
[0113]A test was run to measure the limescale build up control using various commercially available water treatment materials. Two separate tests were run. The first test was a 100 cycle dishmachine test. A door type dishmachine (Hobart AM-15) was used. The selected test apparatus was connected to the inlet water to the dishmachine so that all of the rinse water for the machine was treated. The inlet water had a hardness of 17 grains. Glassware was placed inside the dishmachine in a glassware rack. The machine was run normally for 100 cycles. No chemicals, e.g., detergents, rinse aids, other than the treatment apparatuses were used in this test. After the 100 cycles were complete, the glassware was removed and allowed to air dry. Photos of the glasses were taken. A light box was used to determine reflectance which is a direct correlation to the amount of scale present. The photos and light box scores were compared for the different water treatments tested. A light box score differin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com