Method for synthesising microparticles
a technology of microparticles and synthesis methods, applied in the direction of metal layered products, inorganic chemistry, layered products, etc., can solve the problems of difficult to form high yields of porous particles, short separation of simple mixtures, and limited use of small particles, so as to increase the pore size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
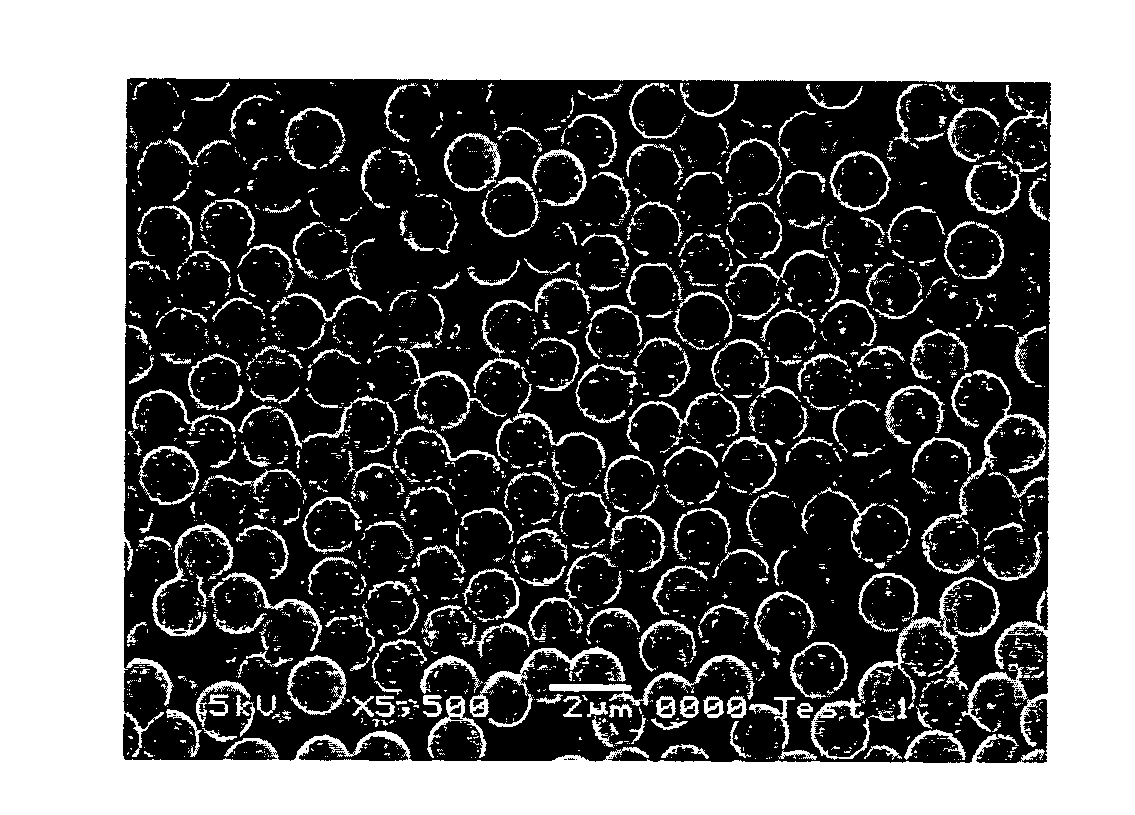
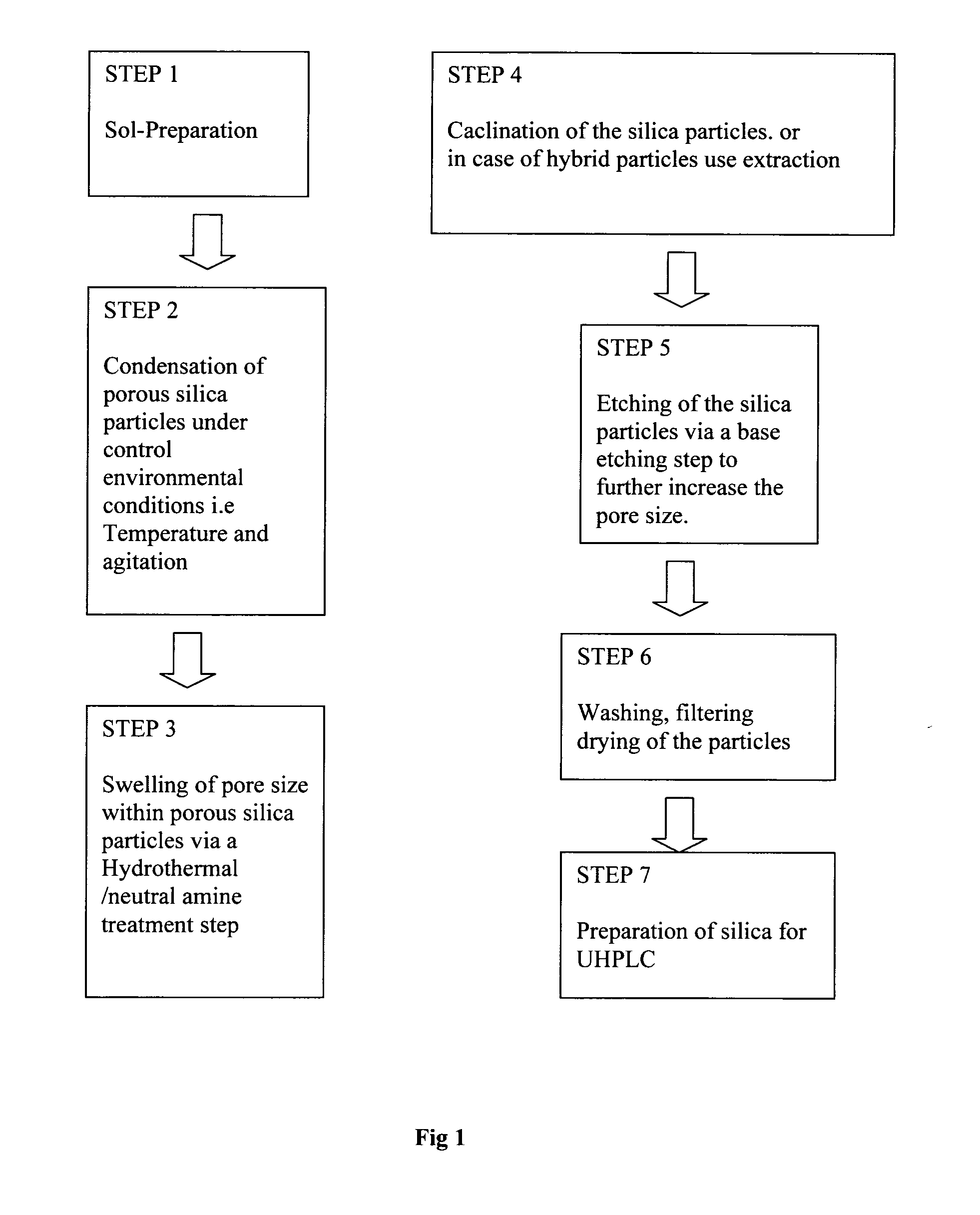
[0128]Mesoporous silica particles are prepared in several stages, as represented schematically in FIG. 1 and described below:
[0129]Step 1: CTAB (about 0.001 to about 0.006 moles, typically about 0.0032 moles) is first dissolved in methanol (at a concentration of about 8 to about 14 moles, typically about 12.36 moles). Ammonia (about 0.05 to about 1.5 moles, typically 0.505 moles) and water (about 2 to about 10 moles, typically about 6.153 moles) are added to the mixture and stirred for 15 minutes before the one step addition of TEOS (about 0.001 to about 0.08 moles, typically about 0.00826 moles. The silica precursor is typically present at a concentration of between about 5 to about 25% v / v of the pre-sol). The sol is allowed to stir for between 24 and 96 hours. The pre-sol solution may be prepared at temperatures between −5 and 80° C. and agitation speed of between 0 and 1000 rpm. The pre-sol solution should be clear and free from any visible particles to produce high quality poro...
example 2
Preparation of Mesoporous Silica Spheres
[0138]Mesoporous silica spheres were prepared based on modified methods described by Shimura et al.12 and Unger et al.15 Tetraethoxysilane (TEOS) was used as the silica precursor, while cetyltrimethylammonium bromide (CTAB) acted as the surfactant template. Methanol (MeOH) was used as the co-solvent.
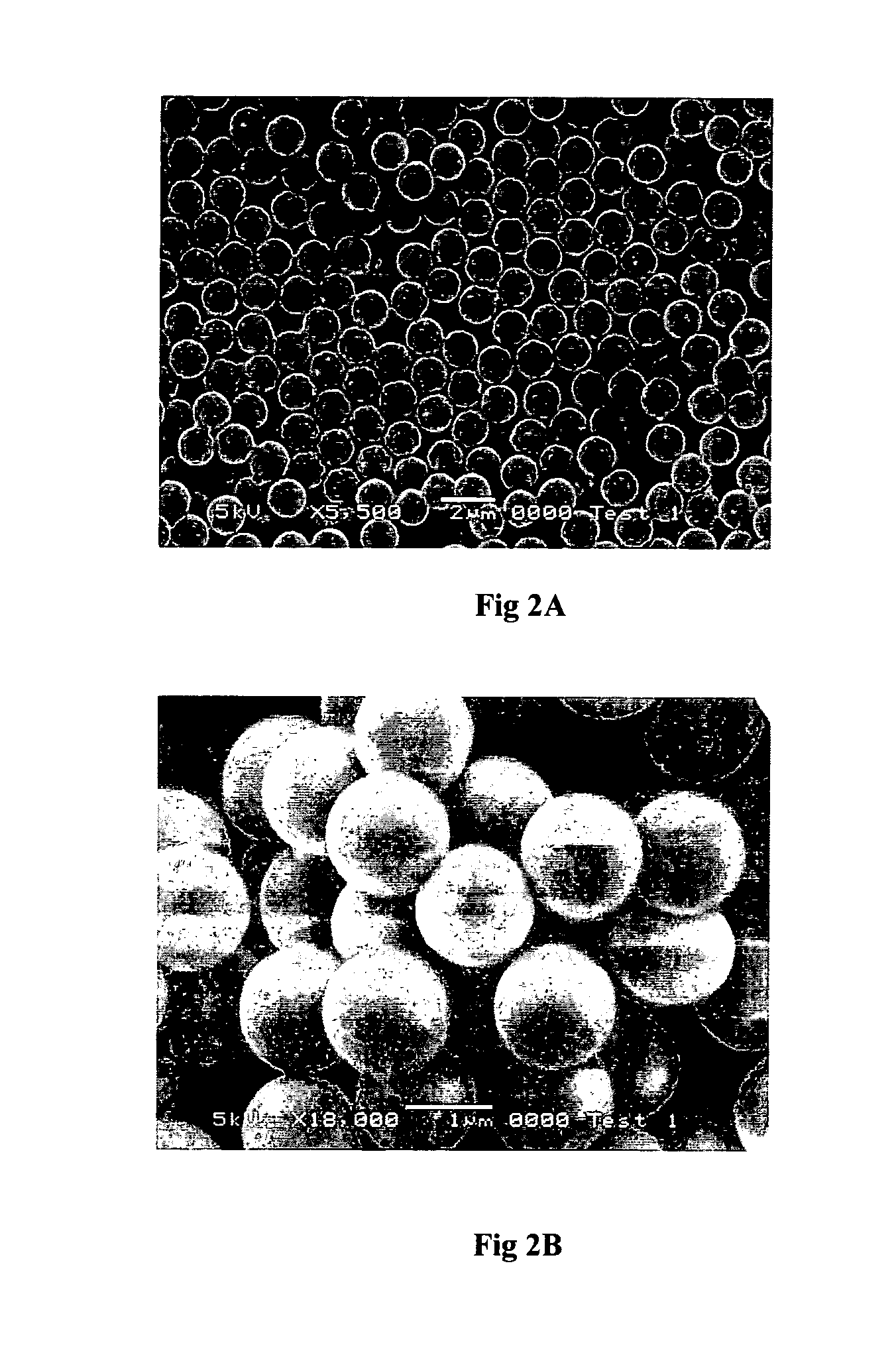
[0139]In a typical reaction, 1.25 g of CTAB was mixed in a 2 L beaker with 88 ml of H2O and 500 ml of methanol and was left stirring (200 rpm) for 10 mins. 32 ml of NH4OH was then added to the solution and the system was left stirring for a subsequent 10 mins. Finally 8 ml of TEOS was added to the solution in a one step addition and the stirring speed increased to 300 rpm. The reaction temperature was controlled at 16° C. The liquid suspension was filtered from the beaker after 24 hours and was subsequently washed with MeOH. It was air-dried at room temperature for 2 hours. A known mass of the as-synthesised material was then added to a pre-prepare...
example 3
Preparation of Mesoporous Silica Spheres
[0140]Mesoporous silica spheres were prepared based on modified methods described by Shimura et al.12 and Unger et al.15 Tetraethoxysilane (TEOS) was used as the silica precursor, while cetyltrimethylammonium bromide (CTAB) acted as the surfactant template. Methanol (MeOH) was used as the co-solvent.
[0141]In a typical reaction, 1.25 g of CTAB was mixed in a 2 L beaker with 88 ml of H2O and 500 ml of methanol and was left stirring (200 rpm) for 10 mins. 32 ml of NH4OH was then added to the solution and the system was left stirring for a subsequent 10 mins. Finally 8 ml of TEOS was added to the solution in a one step addition and the stirring speed increased to 300 rpm. The reaction temperature was controlled at 16° C. The liquid suspension was filtered from the beaker after 24 hours and was subsequently washed with MeOH. It was air-dried at room temperature for 2 hours. A known mass of the as-synthesised material was then added to a pre-prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com