Novel chondroitin sulfate having decreased molecular weight and use thereof
a chondroitin sulfate and molecular weight technology, applied in the direction of drug compositions, antinoxious agents, extracellular fluid disorder, etc., can solve the problems of deterioration of peritoneal function, poor water removal or insufficient removal of waste products, and achieve the effect of reducing molecular weigh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Chondroitin Sulfate Having Decreased Molecular Weight of the Present Invention (1)
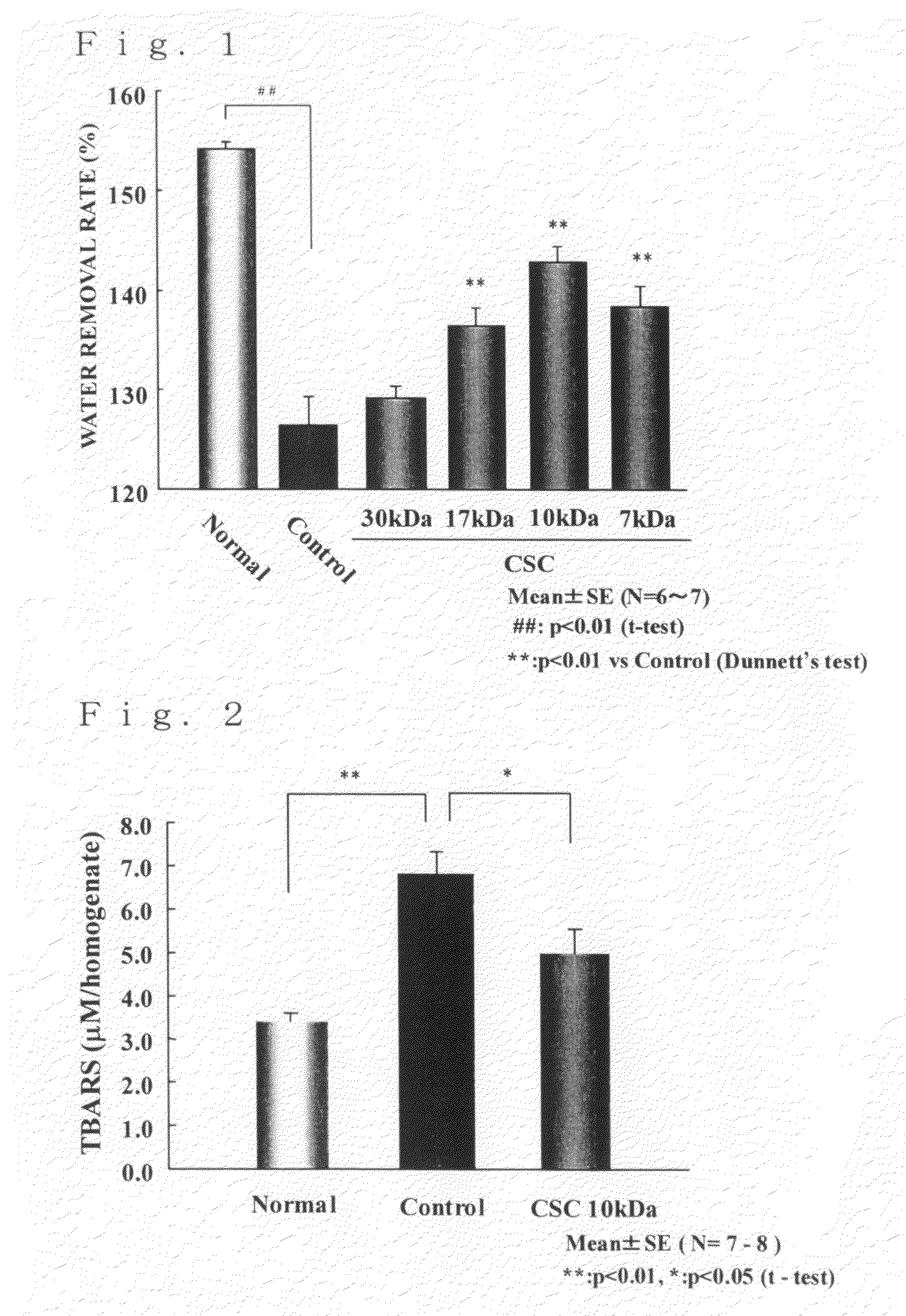
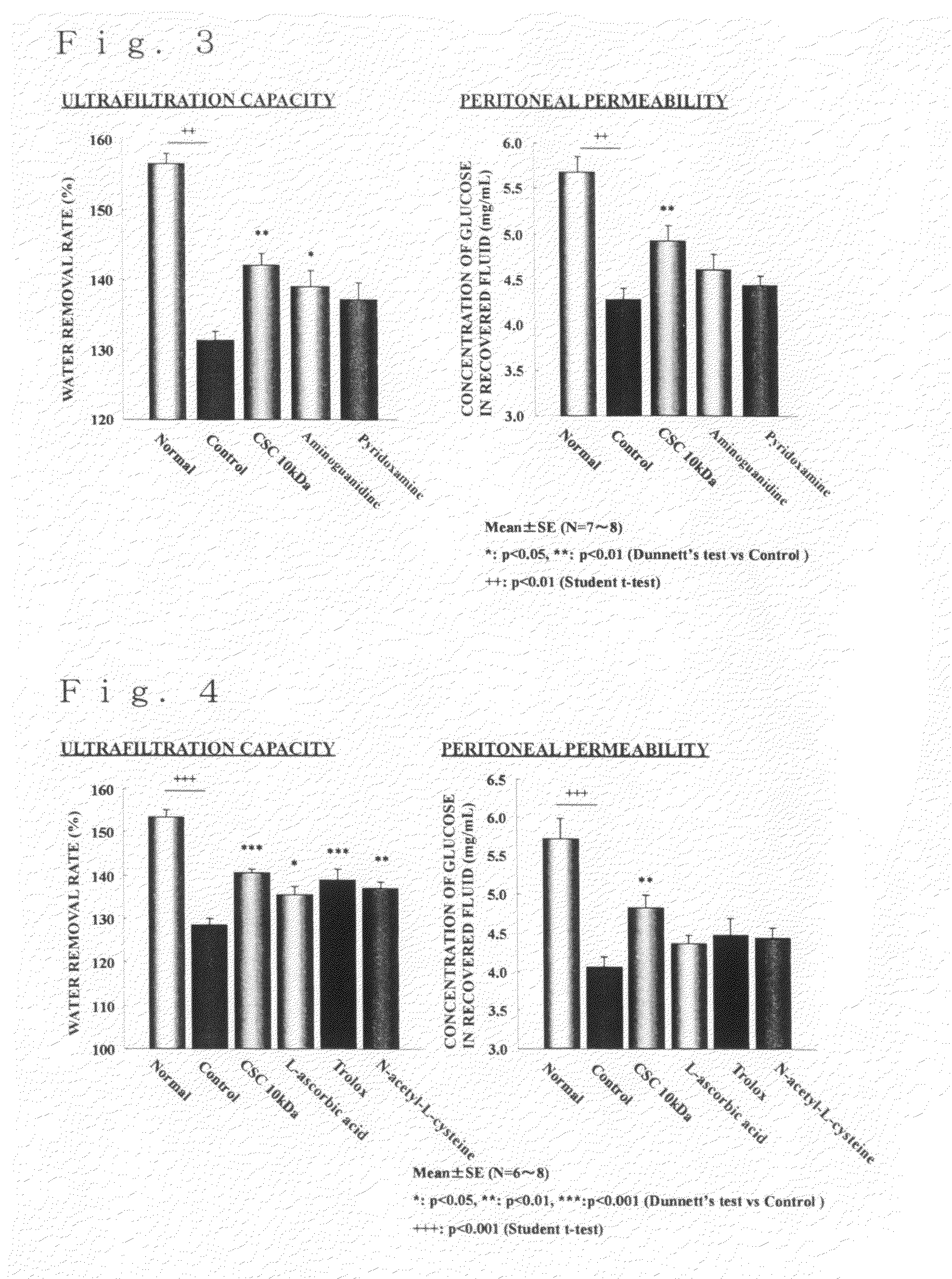
[0027]Prionace glauca-derived chondroitin sulfate C (weight average molecular weight: 30 kDa, trade name of Seikagaku Corporation: Chondroitin sulfate C, sodium salt (shark cartilage), SG) was used as a starting material and dissolved in an amount of 1 g in 50 mL of PBS (pH 5.3). To this solution, 100,000 U of ovine testicular hyaluronidase (manufactured by Sigma, type V) was added, and the enzymatic reaction was allowed to proceed at 37 ° C. A portion of the reaction mixture was taken over time and analyzed by GPC-HPLC to examine the degree of reduction of the molecular weight. When a desired molecular weight was obtained, the reaction mixture was boiled to stop the enzymatic reaction. In the case where the desired molecular weight was not obtained, ovine testicular hyaluronidase was further added to the reaction mixture and the enzymatic reaction was allowed to proceed. When the desire...
example 2
Preparation of Chondroitin Sulfate Having Decreased Molecular Weight of the Present Invention (2)
[0029]Prionace glauca-derived chondroitin sulfate C (weight average molecular weight: 30 kDa, trade name of Seikagaku Corporation: Chondroitin sulfate C, sodium salt (shark cartilage), SG) was used as a starting material and subjected to electron beam irradiation at an irradiation energy of 200 kGy according to the method described in JP-A-2004-43645, whereby a chondroitin sulfate having a decreased molecular weight of the present invention having a weight average molecular weight of 10 kDa and containing a constituent disaccharide unit represented by -[4GlcAβ1-3GalNAc(6S)β1]- (wherein GlcA represents a D-glucuronic acid residue; GalNAc represents an N-acetyl-D-galactosamine residue; p1-3 represents a β1-3 glycosidic linkage; β1-4 represents a β1-4 glycosidic linkage; and (6S) indicates that position 6 of the monosaccharide residue is sulfated) in an amount of 73.0% (molar ratio) of the ...
example 3
Effect of Protecting Peritoneum in Peritoneal Dialysis of Chondroitin Sulfate Having Decreased Molecular Weight of the Present Invention (1)
(Experimental Method)
[0030]To a Wistar male rat at 8 weeks of age, Midpeliq 250 (trade name, a peritoneal dialysis fluid containing glucose at a concentration of 2.5% (w / v) manufactured by Terumo Corporation) in which a test substance was dissolved at a concentration of 0.1% (w / v) was repetitively administered into the peritoneal cavity once daily at 15 mL / body for 7 days under ether anesthesia. To a control group (Control), Midpeliq 250 was administered in the same manner. On the next day after the final administration, a peritoneal equilibration test was performed, and the peritoneal function was evaluated. To be more specific, Midpeliq 250 was injected into the peritoneal cavity at 60 mL / kg, and 4 hours thereafter, the fluid remaining in the peritoneal cavity was recovered. The amount of the recovered fluid was measured, and the ultrafiltrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com