Medical Devices Incorporating Carbon Nanotube Material and Methods of Fabricating Same
a carbon nanotube and material technology, applied in the field of medical devices, can solve the problems of high biocompatibility, and achieve the effects of low weight, high strength, and stable sta
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
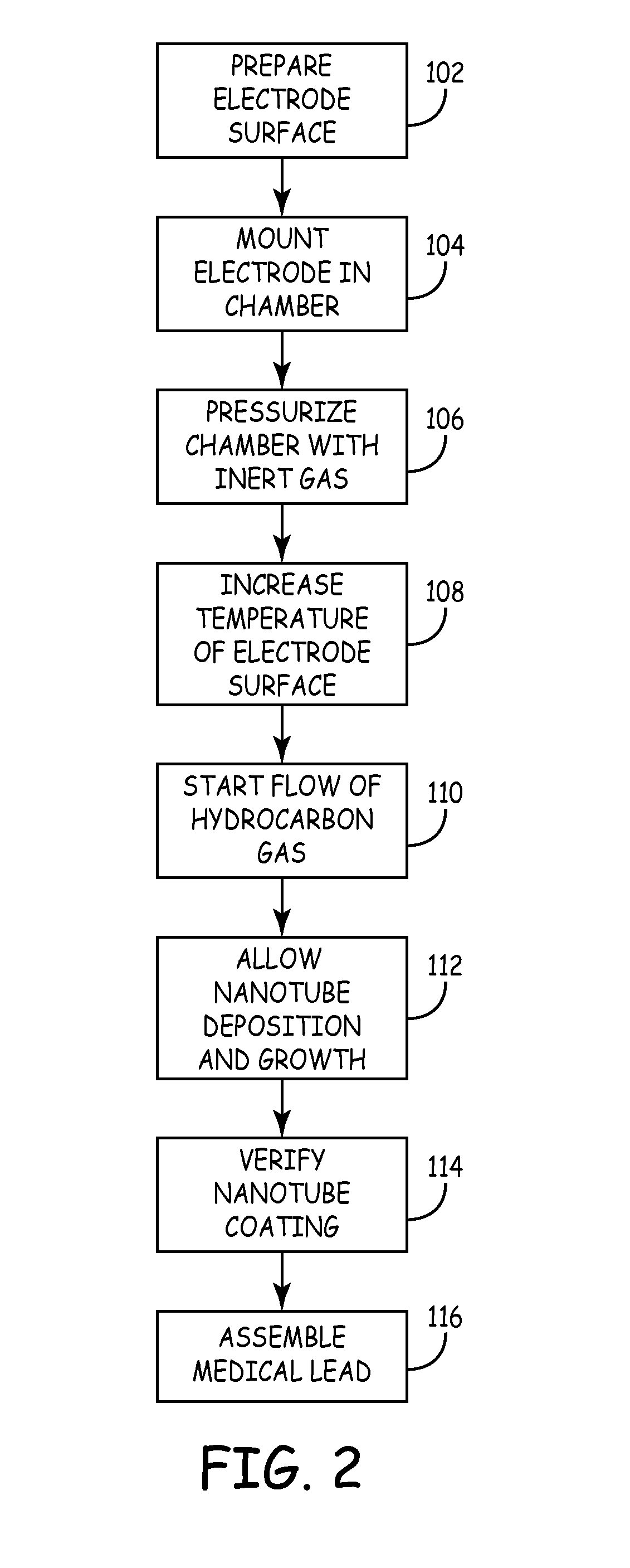
Method used
Image
Examples
Embodiment Construction
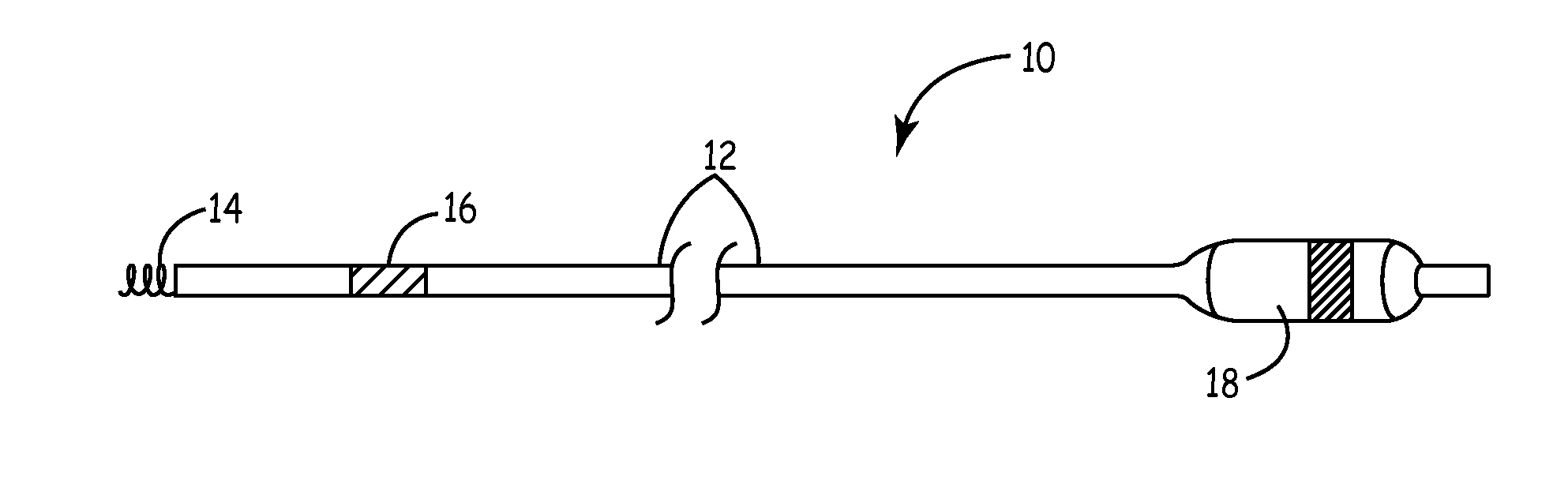
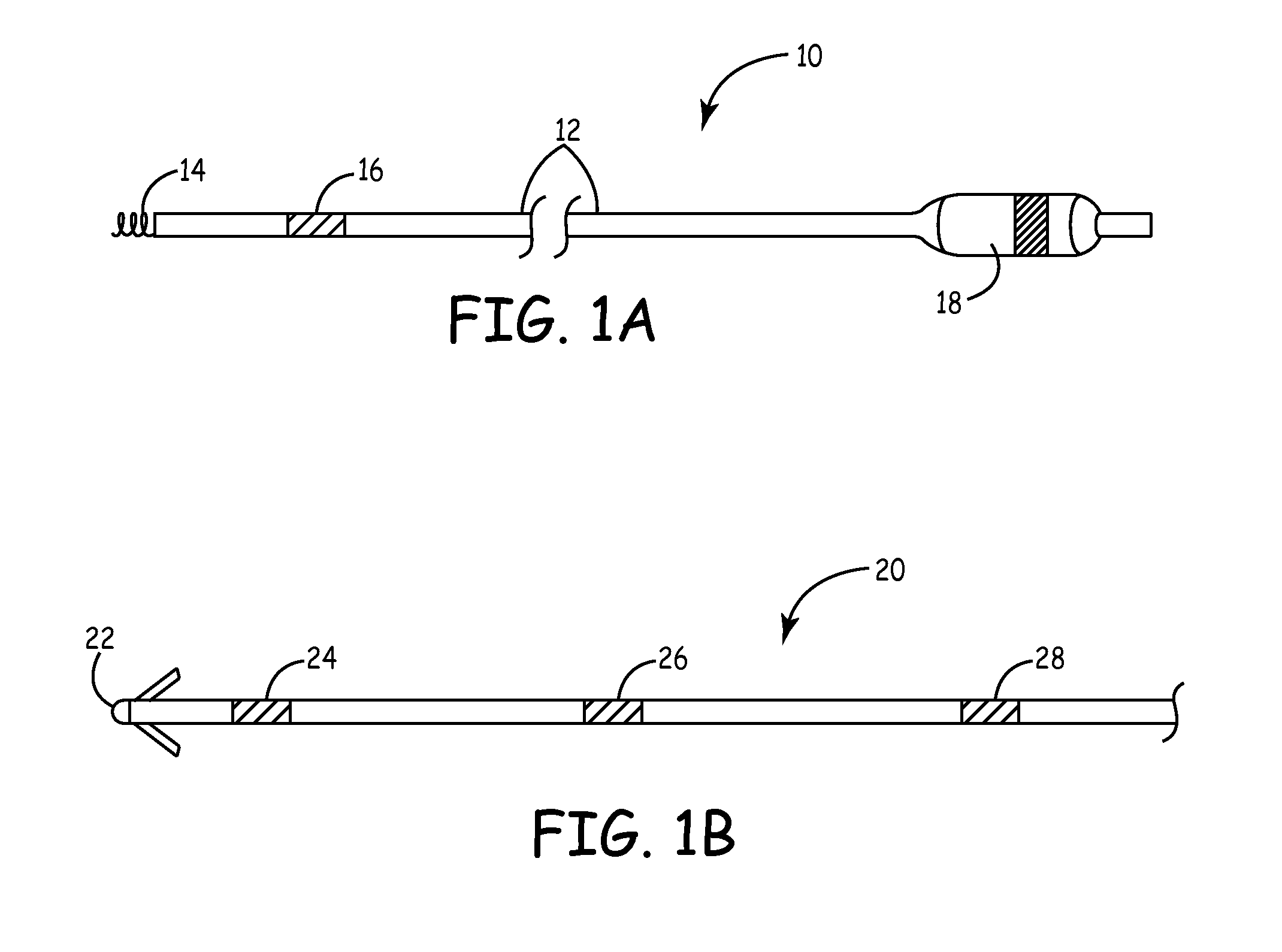
[0032]As described above, the present invention is directed at providing a medical lead having improved electrode performance by providing carbon nanotube coated electrodes. FIGS. 1A and 1B depict exemplary medical leads of the type that may be used with the present invention. FIG. 1A is a plan view of a medical lead 10 that may typically be used for cardiac pacing and / or sensing. Lead 10 is provided with an elongated lead body 12, a helical tip electrode 14 located at the distal end of the lead and a ring electrode 16 spaced proximally from tip electrode 14. A connector assembly 18 at the proximal end of lead 10 is used to connect the lead to a medical device, such as a pacemaker. Conductors extending the length of lead body 12 electrically couple the tip electrode 14 and ring electrode 16 to respective connectors carried by the connector assembly 18.
[0033]FIG. 1B is a plan view of the distal end of a medical lead 20 of the type that may be used for pacing, sensing, cardioversion a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com