Interleukin-1 Conjugates and Uses Thereof
a technology of interleukin-1 and conjugates, applied in the field of medicine, public health, immunology, molecular biology and virology, can solve the problems of high cost of goods, potential patient compliance problems, kineret® prescribing information, etc., to inhibit the development of atherosclerosis symptoms, reduce the pro-inflammatory activity of il-1 in vivo, and improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning, Expression and Purification of Murine IL1α117-270 and IL-1β119-269
[0156]The nucleotide sequence encoding amino acids 117-270 of murine IL-1α was amplified by PCR from a cDNA library of TNFα-activated murine macrophages using oligonucleotides IL1α1 (5′-ATATATGCTAGCCCCTTACACCTACCAGAGTGATTTG-3′; SEQ ID NO:24) and IL1α2 (5′-ATATATCTCGAGTGATATCTGGAAGTCTGTCATA GAG-3′; SEQ ID NO:25). Using the same cDNA library, the nucleotide sequence encoding amino acids 119-269 of the murine IL-1β precursor was amplified with oligonucleotides IL1β1 (5′-ATATATGCTAGCCCCCATTAGACAGCTGCACTACAGG-3′; SEQ ID NO:26) and IL1β2 (5′-ATATATCTCGAGGGAAGACACAGATTCCATGGTGAAG-3′; SEQ ID NO: 27). Both DNA fragments were digested with NheI and XhoI, and cloned into the expression vector pModEC1 (SEQ ID NO:29)
[0157]The vector pModEC1 (SEQ ID NO:29) is a derivative of pET22b(+) (Novagen Inc.), and was constructed in two steps. In a first step the multiple cloning site of pET22b(+) was changed by replacing the origi...
example 2
A. Coupling of Mouse IL-1β119-269 to Qβ Virus-like Particles
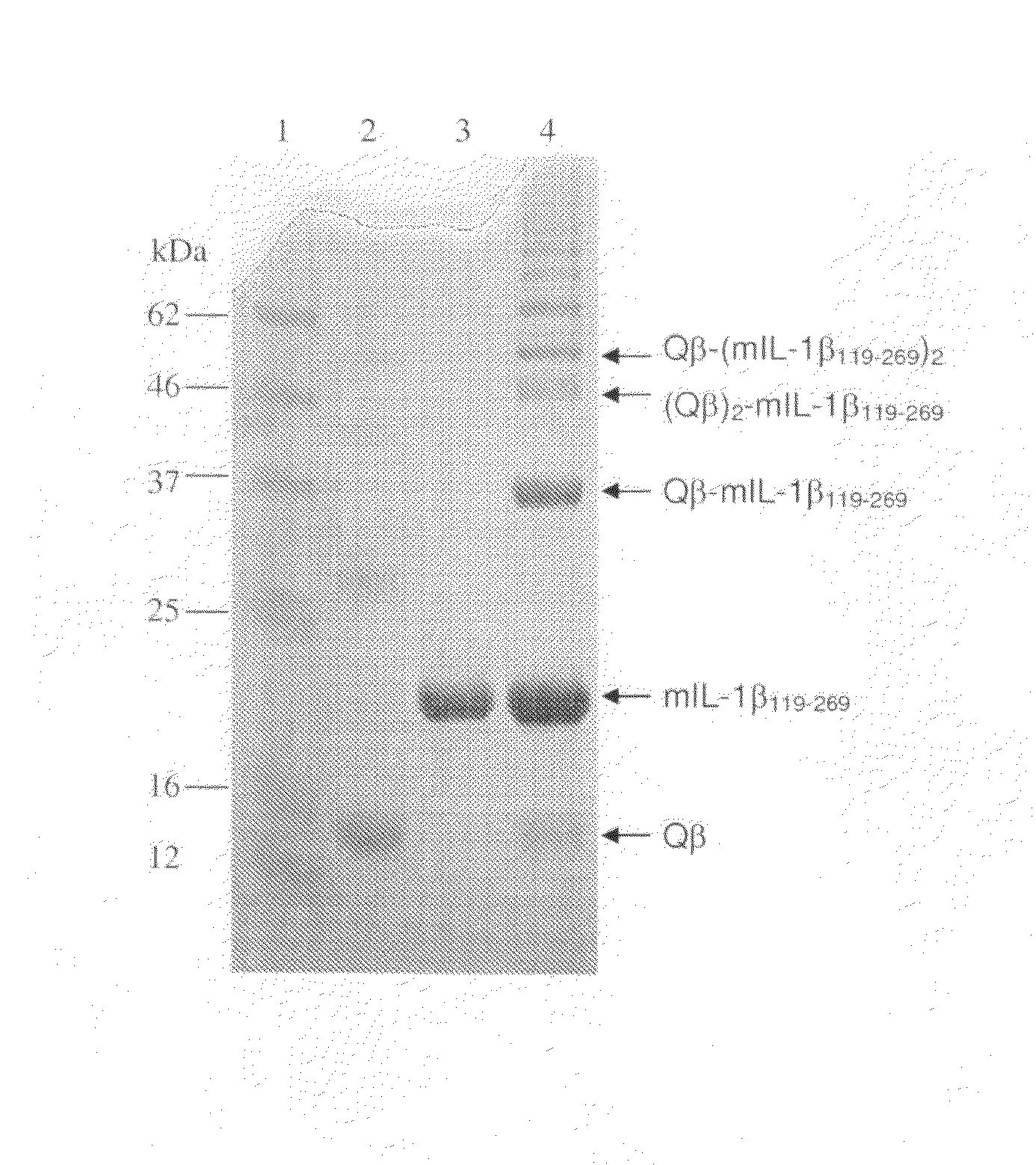
[0159]A solution containing 1.3 mg / ml of the purified murine IL-1β119-269 protein from EXAMPLE 1 (SEQ ID NO:66) in PBS pH 7.2 was incubated for 60 min at room temperature with an equimolar amount of TCEP for reduction of the C-terminal cysteine residue.
[0160]A solution of 6 ml of 2 mg / ml Qβ capsid protein in PBS pH 7.2 was then reacted for 60 min at room temperature with 131 μl of a SMPH solution (65 mM in DMSO). The reaction solution was dialysed at 4° C. against three 3 l changes of 20 mM HEPES, 150 mM NaCl pH 7.2 over 24 hours. Seventy-five μl of the derivatized and dialyzed Qβ solution was mixed with 117 μl H2O and 308 μl of the purified and pre-reduced mouse IL-1β119-269 protein and incubated over night at 15° C. for chemical crosslinking. Uncoupled protein was removed by tangential flow filtration against PBS using cellulose ester membranes with a molecular weight cutoff of 300.000 Da.
[0161]Coupled products were analy...
example 3
A. Coupling of Mouse IL-1α117-270 to Qβ Virus-like Particles
[0169]A solution containing 1.8 mg / ml of the purified IL-1α117-270 protein from EXAMPLE 1 (SEQ ID NO:65) in PBS pH 7.2 was incubated for 60 min at room temperature with an equimolar amount of TCEP for reduction of the C-terminal cysteine residue.
[0170]A solution of 6 ml of 2 mg / ml Qβ capsid protein in PBS pH 7.2 was then reacted for 60 minutes at room temperature with 131 μl of a SMPH solution (65 mM in DMSO). The reaction solution was dialyzed at 4° C. against three 3 l changes of 20 mM HEPES, 150 mM NaCl pH 7.2 over 24 hours. Seventy-five μl of the derivatized and dialyzed Qβ solution was mixed with 192 μl H2O and 233 μl of the purified and pre-reduced mouse IL-1α117-270 protein and incubated over night at 15° C. for chemical crosslinking. Uncoupled protein was removed by tangential flow filtration against PBS using cellulose ester membranes with a molecular weight cutoff of 300.000 Da.
[0171]Coupled products were analyzed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com