Targeted Coagulation Factors and Method of Using the Same
a technology of coagulation factors and target coagulation, which is applied in the direction of fusions for specific cell targeting, peptide/protein ingredients, extracellular fluid disorder, etc. it can solve the problems of limited effect of biological drugs, and limited treatment options for hemophilia patients with fviii concentrates or recombinant fviii, etc., to achieve enhanced protein therapeutic effect and high local concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
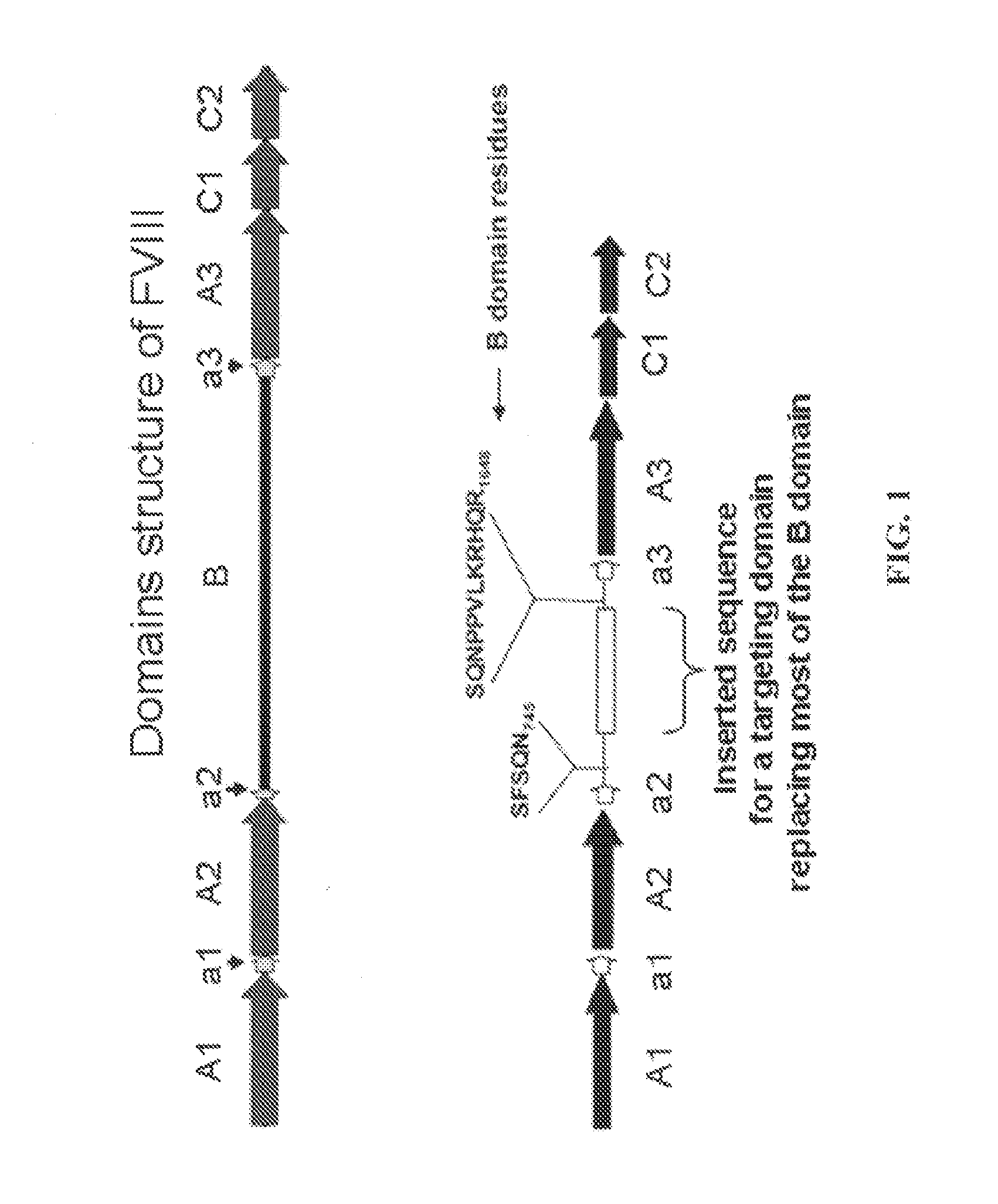
[0054]Modified RGD Peptides with High Affinity for GPIIb / IIIa Binding
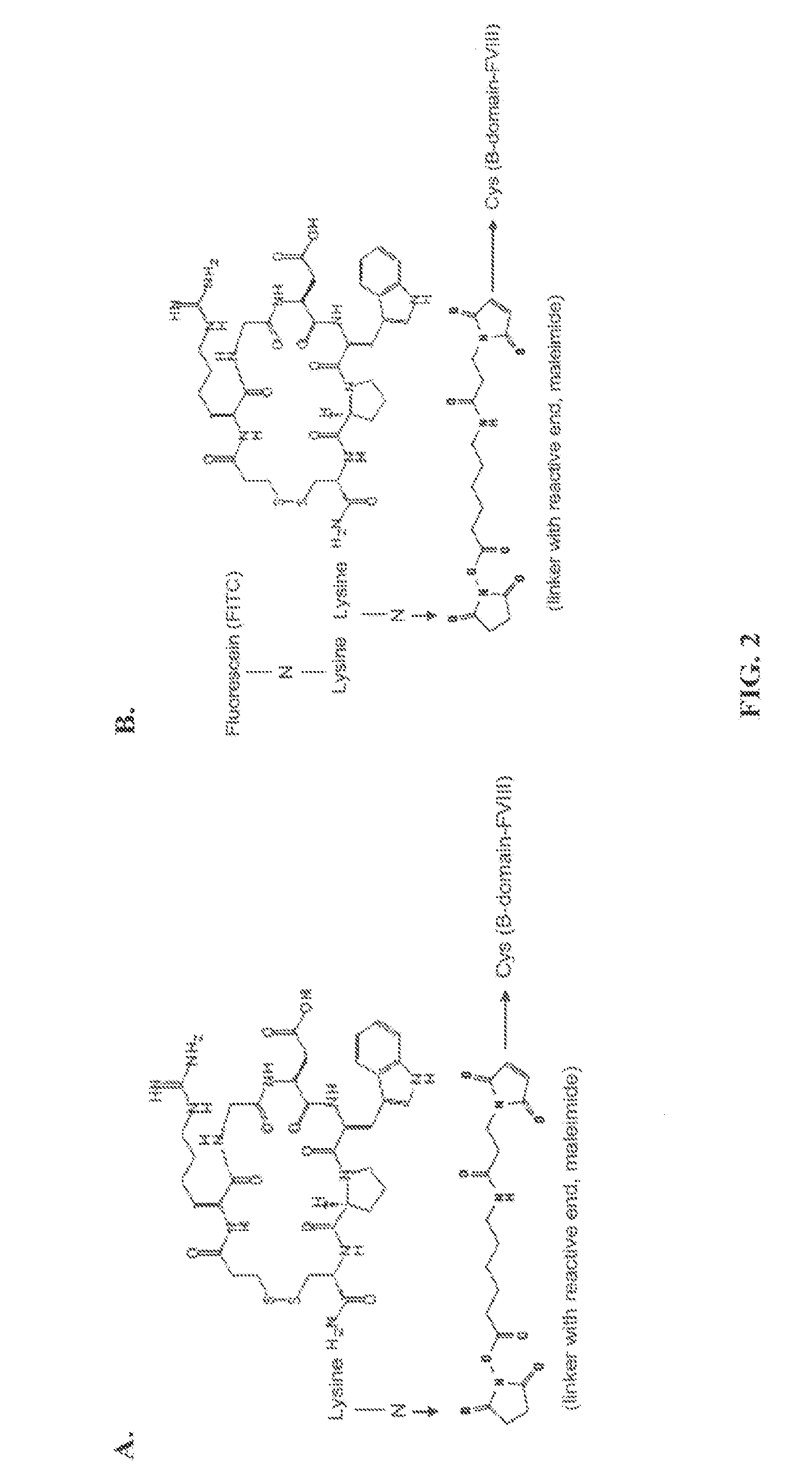
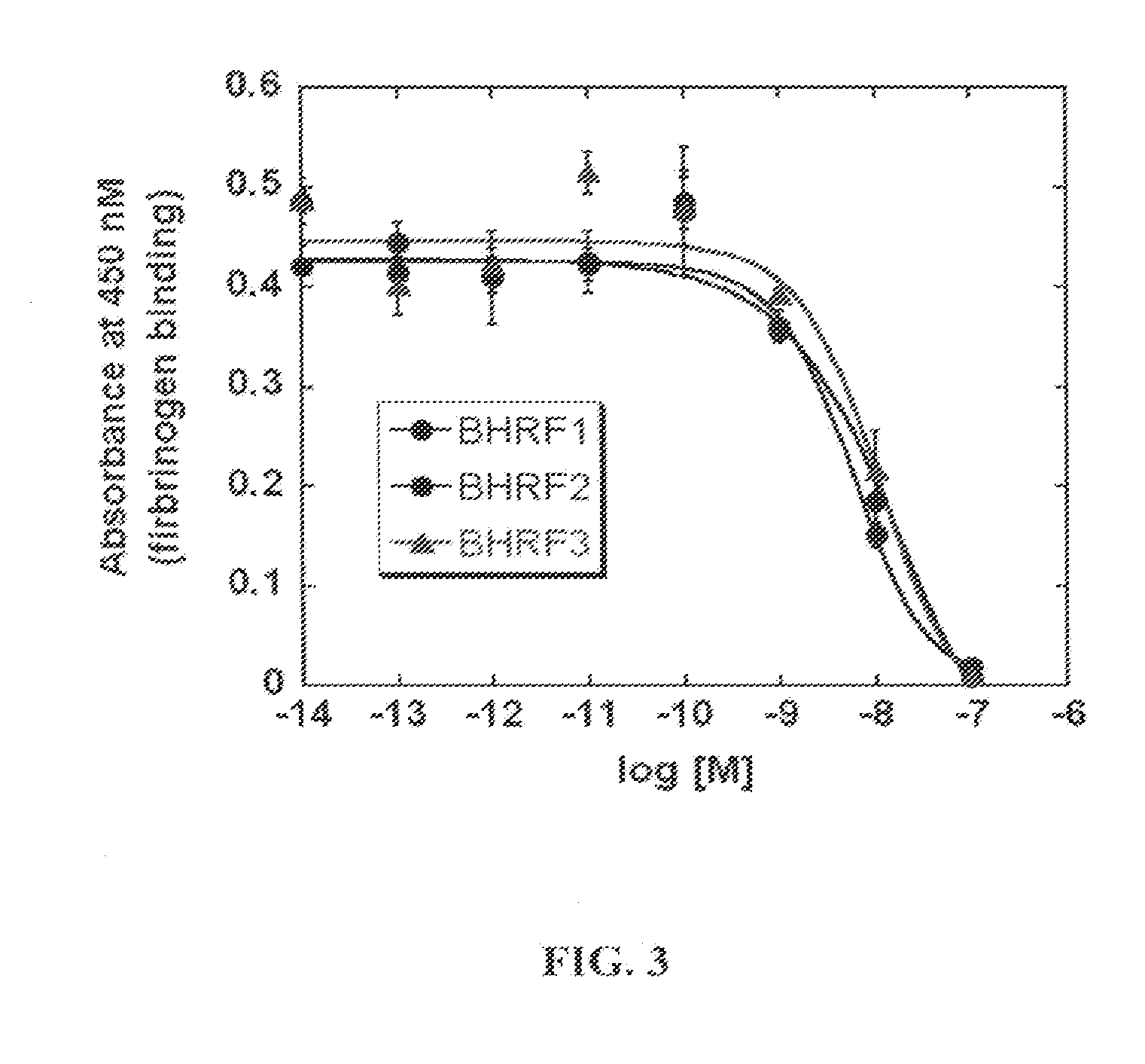
[0055]Cyclic peptides have been described to bind potently and selectively to GPIIb / IIIa. One such peptide, integrilin, was used as a targeting domain to link with FVIII as it has been shown that integrilin can selectively bind to GPIIb / IIIa. Integrilin was modified by adding a short PEG linker ending in a maleimide moiety that can selectively couple to free cysteine residues in proteins. The modified integrilin is termed BHRF-1 with the linker only (FIG. 2A), and BHRF-3 with the linker and a fluorescein (FITC) (FIG. 2B). As shown in FIG. 3, the modified integrilins retain affinity for GPIIb / IIIa as they potently blocked fibrinogen (Fbn) binding to immobilized GPIIa / IIIb.
[0056]Peptide binding to GPIIb-IIIa was measured using a solid phase binding assay in which competition of fibrinogen binding by testing compounds is measured. The assay was performed as follows. Purified GPIIb-IIIa (Innovative Research, Novi, Mich...
example 2
[0058]Coupling GPIIb / IIIa Binding Peptides to FVIII
[0059]The polypeptide sequence of the full-length FVIII is known in the art (see, e.g., SEQ ID NO: 1, SEQ ID NO: 2, and as disclosed in WO 2006 / 053299.
Concentration of FVIII and uncapping of free sulfhydryl groups
[0060]The Cys residue located in the B-domain of recombinant FVIII can be capped by cysteine present in the media during protein expression, but it can be readily removed by treatment with reducing agents, such as TCEP, as follows. FVIII (20 mL) was thawed and concentrated in two Amicon®-15 cartridges (Millipore, Billerica, Mass.), spun at 2000×g (about 3153 rpm) for 25 minutes in the cold. The concentration of the 2.8 mL retentate is about 0.8-0.9 mg / mL by A280 using a NanoDrop® spectrophotometer (ThermoFisher Scientific, Waltham, Mass.). The buffer was then exchanged using a 10 mL Zeba desalting cartridge, pre-equilibrated with 50 mM Tris, 150 mM NaCl, 2.5 mM CaCl2 and 100 ppm Tween®-80 (polyoxyethylenesorbitan monooleate...
example 3
[0064]BHRF-1-FVIII Binds to Immobilized GPIIb / IIIa
[0065]To test the binding activity of BHRF-1-FVIII to GPIIb / IIIa, biotinylated GPIIb / IIIa was immobilized on streptavidin plates and treated with either BHRF-1-FVIII or unmodified FVIII, both in binding buffer (50 mM Tris, pH 7.5, 100 mM NaCl2, 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2 and 1 mg / mL BSA). The unbound protein was removed by washing three times with binding buffer. Assay buffer (25 μL) was added to the plate, and FVIII activity was determined using a chromogenic assay kit (Coatest® SP4, Chromogenix, Lexington, Mass.). As shown in FIG. 4, there was binding of BHRF-1-FVIII, while only little binding of unmodified FVIII was detected. The increased binding of BHRF-1-FVIII was completely eliminated by addition of a cyclic RGD peptide (GpenGRGDSPCA; SEQ ID NO: 5) that competes for BHRF-1 binding to GPIIb / IIIa. Furthermore, only low background levels of either protein bound when no GPIIb / IIIa was immobilized on the plate. These data s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com