Novel process for the manufacture of pharmaceutical preparations
a technology of solid dispersions and pharmaceutical preparations, which is applied in the direction of biocide, muscular disorder, drug compositions, etc., can solve the problems of poor bioavailability, low dissolution rate, and poor bioavailability of poorly bioavailable compounds, so as to improve stability, reduce the amount of organic solvents, and improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
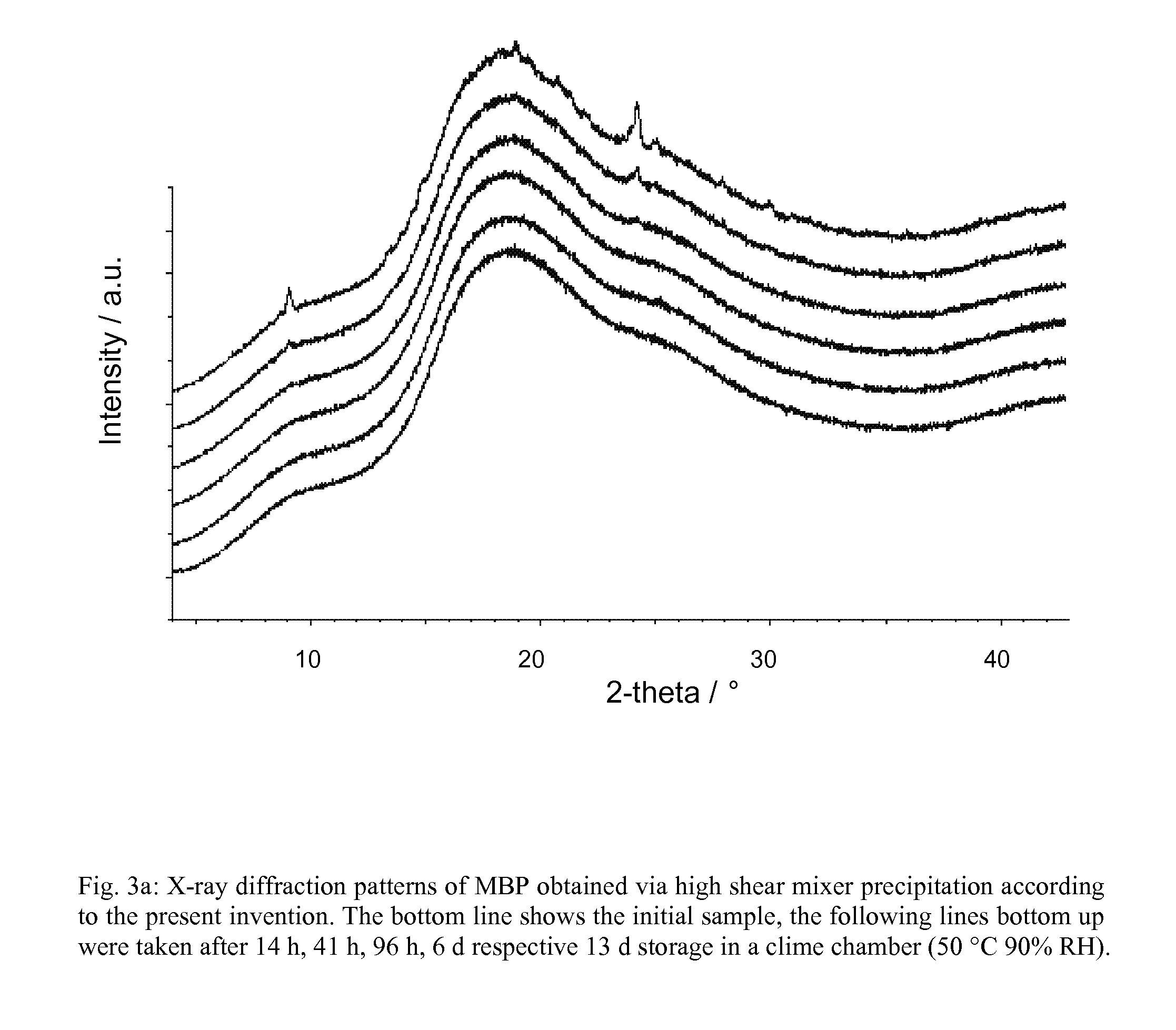
Examples
example 1
Preparation of the DMA Phase
[0050]The concentration of the compound of formula 1 and HPMCAS in the organic solvent was 35% (w / w), while the ratio of the compound of formula 1 and HPMCAS is 30 to 70: The temperature of the solution was adjusted to 70° C.
[0051]In a 250 ml double jacked glass flask reactor 21 g of the compound of formula 1 were dissolved in 130 g Dimethylacetamide (DMA) at 20-25° C. Under stirring, 48.9 g of HPMC-AS were added to the solution. The mixture was heated up to 70° C. A clear solution was obtained.
example 2
Preparation of the Aqueous Phase
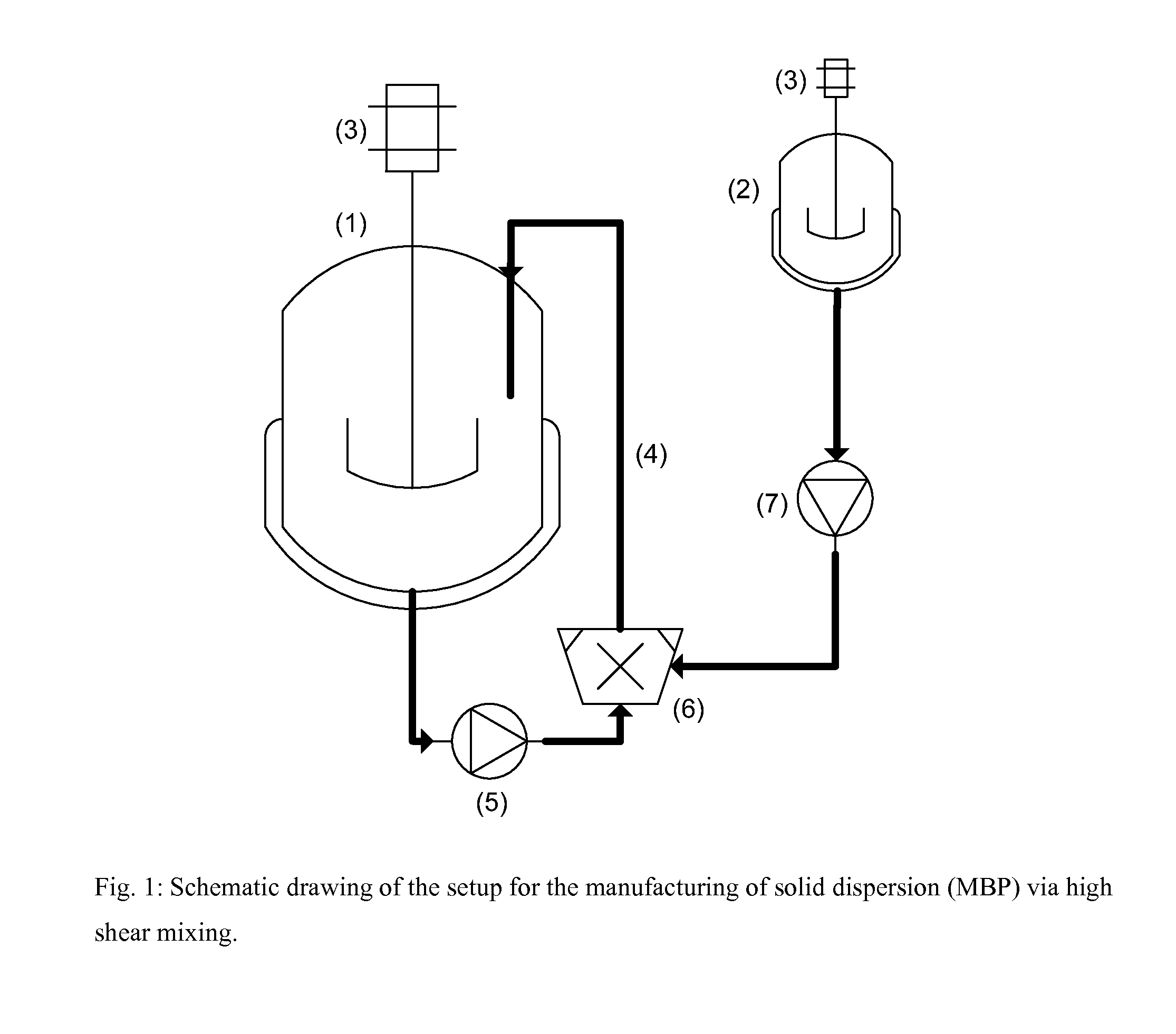
[0052]In a double jacketed 2.0 liter reactor 1210 g of 0.01 N HCl was tempered to 5° C. Out of the bottom valve of the reactor the water phase was circulated by the high shear mixer or with an auxiliary pump, preferred a rotary lobe pump, and then followed by the high shear mixer, back to the top of the reactor. The inlet of the recirculation into the reactor was under the fluid level in order to prevent foaming (see FIG. 1).
example 3
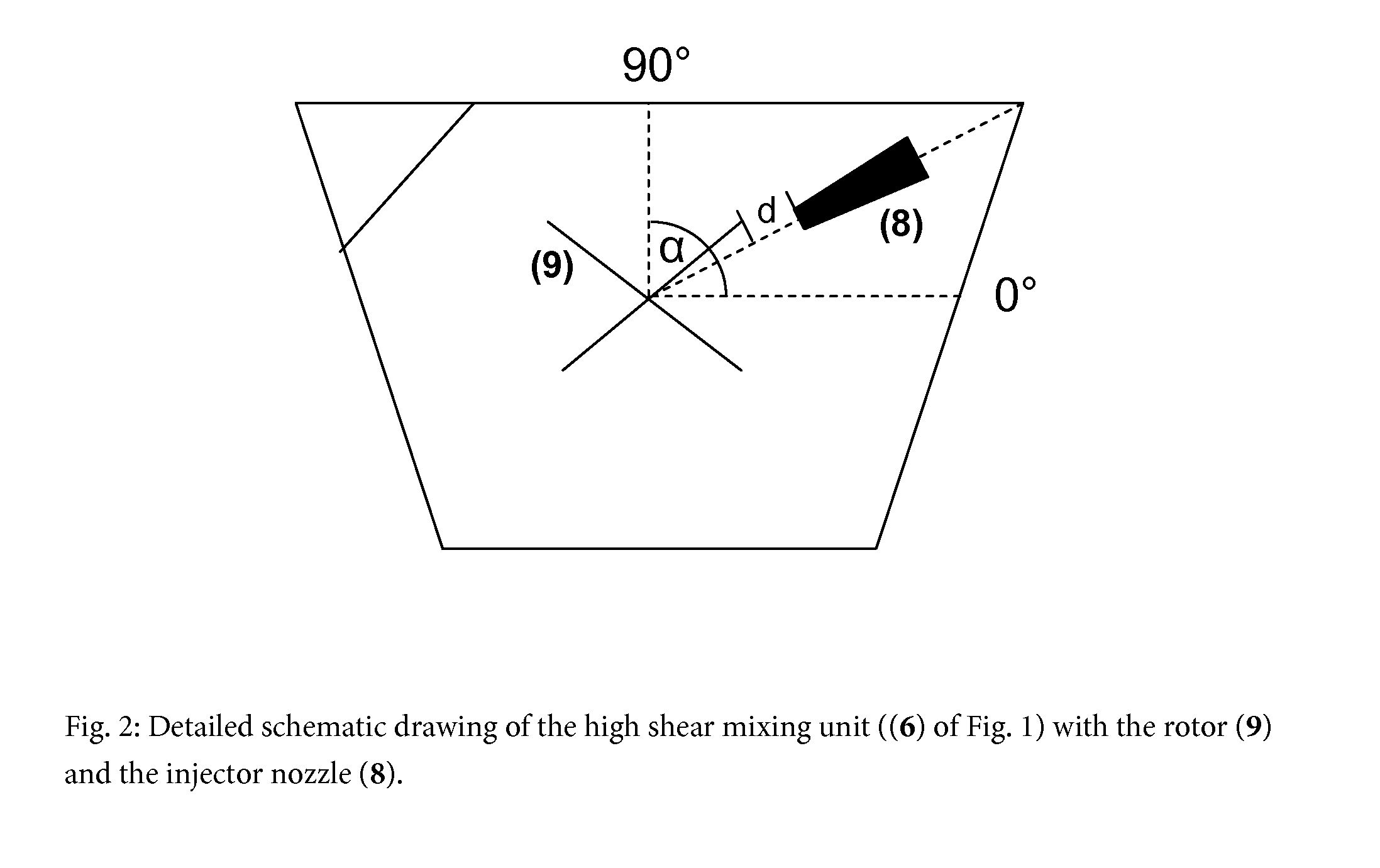
[0053]The tip speed of the rotor in the high shear mixer was set 25 m / sec. A rotor / stator combination with one teeth row, each for rotor and stator was used.
Dosing of the DMA Solution
[0054]The DMA solution tempered at 70° C. was dosed with a gear pump via an injector nozzle, which was pointing into the mixing chamber of the high shear mixer, into the circulating aqueous phase.
Dosing Rate of the DMA Solution
[0055]The DMA solution was dosed into the aqueous phase resulting in a ratio of HCl / DMA, in the mixing chamber of the high shear mixer of 100 / 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| speed | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com