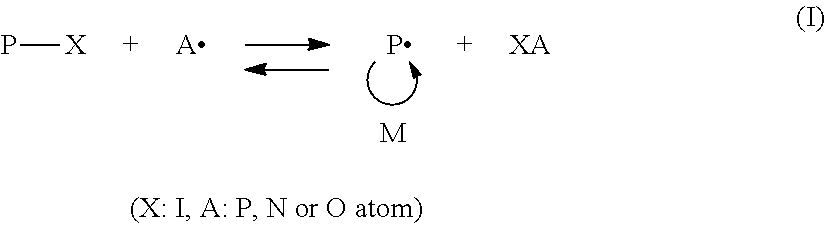Pigment dispersions, block polymers and manufacturing method therefor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of A-B Block Polymer Having Glycidyl Groups Reacted with Di-n-butylamine
[0107]Into a reaction vessel fitted with a stirrer, reflux condenser, thermometer and nitrogen inlet tube, propylene glycol monomethyl ether acetate (hereinafter abbreviated as “PGMAc”) (100 parts), iodine (3.0 parts), 2,2′-azobis(isobutyronitrile) (hereinafter abbreviated as “AIBN”) (5.9 parts), methyl methacrylate (hereinafter abbreviated as “MMA”) (75 parts) and diethyl phosphite (hereinafter abbreviated as “DEP”) (0.766 parts) were charged, and with nitrogen bubbling, polymerization was conducted at 80° C. for 2 hours to form an A block. The polymerization mixture was sampled, and its solid content was measured. The polymerization conversion rate as calculated from the nonvolatile content was 100%. Further, Mn as measured by a visible light detector (hereinafter abbreviated as “RI”) of GPC was 2,700, and PDI was 1.22.
[0108]Glycidyl methacrylate (hereinafter abbreviated as “GMA”) (17.8 parts) was th...
synthesis example 2
Synthesis of A-B Block Polymer Having Glycidyl Groups Reacted with Diphenylamine
[0110]Into a similar reaction vessel as in Synthesis Example 1, PGMAc (150 parts), iodine (3.0 parts), AIBN (5.9 parts), benzyl methacrylate (hereinafter abbreviated as “BzMA”) (132.2 parts) and N-iodosuccinimide (hereinafter abbreviated as “NIS”) (0.067 parts) were charged, and with nitrogen bubbling, polymerization was conducted at 80° C. for 3 hours to form an A block. The polymerization mixture was sampled, and its solid content was measured. The polymerization conversion rate as calculated from the nonvolatile content was 100%. Mn as measured by RI at that time was 5,400, and PDI was 1.24. An absorption aromatic ring was observed when measured by an ultraviolet detector (measurement wavelength: 254 nm, hereinafter abbreviated as “UV”). Mn as measured by UV was 5,300, and PDI was 1.25.
[0111]GMA (35.6 parts) was then added, and polymerization was conducted further at the same temperature for 3 hours t...
synthesis example 3
Synthesis of A-B Block Polymer Having Glycidyl Groups Reacted with Di-n-butylamine
[0113]Into a similar reaction vessel as in Synthesis Example 1, PGMAc (250 parts), iodine (6.0 parts), AIBN (10.8 parts), n-butyl methacrylate (hereinafter abbreviated as “BMA”) (213.3 parts) and NIS (0.134 parts) were charged, and with nitrogen bubbling, polymerization was conducted at 80° C. for 3 hours to form an A block. The polymerization mixture was sampled, and its solid content was measured. The polymerization conversion rate as calculated from the nonvolatile content was 100%. Mn as measured by RI of GPC at that time was 4,000, and PDI was 1.26.
[0114]GMA (42.6 parts) was then added, and polymerization was conducted further at the same temperature for 3 hours to form a D block. The polymerization mixture was sampled, and its solid content was measured. The polymerization conversion rate as calculated from the nonvolatile content was 99%. Mn as measured at that time by RI of GPC was 5,500, and P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com