Anode and a method of manufacturing an anode
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example
Preparation of an Anode Material of the Invention
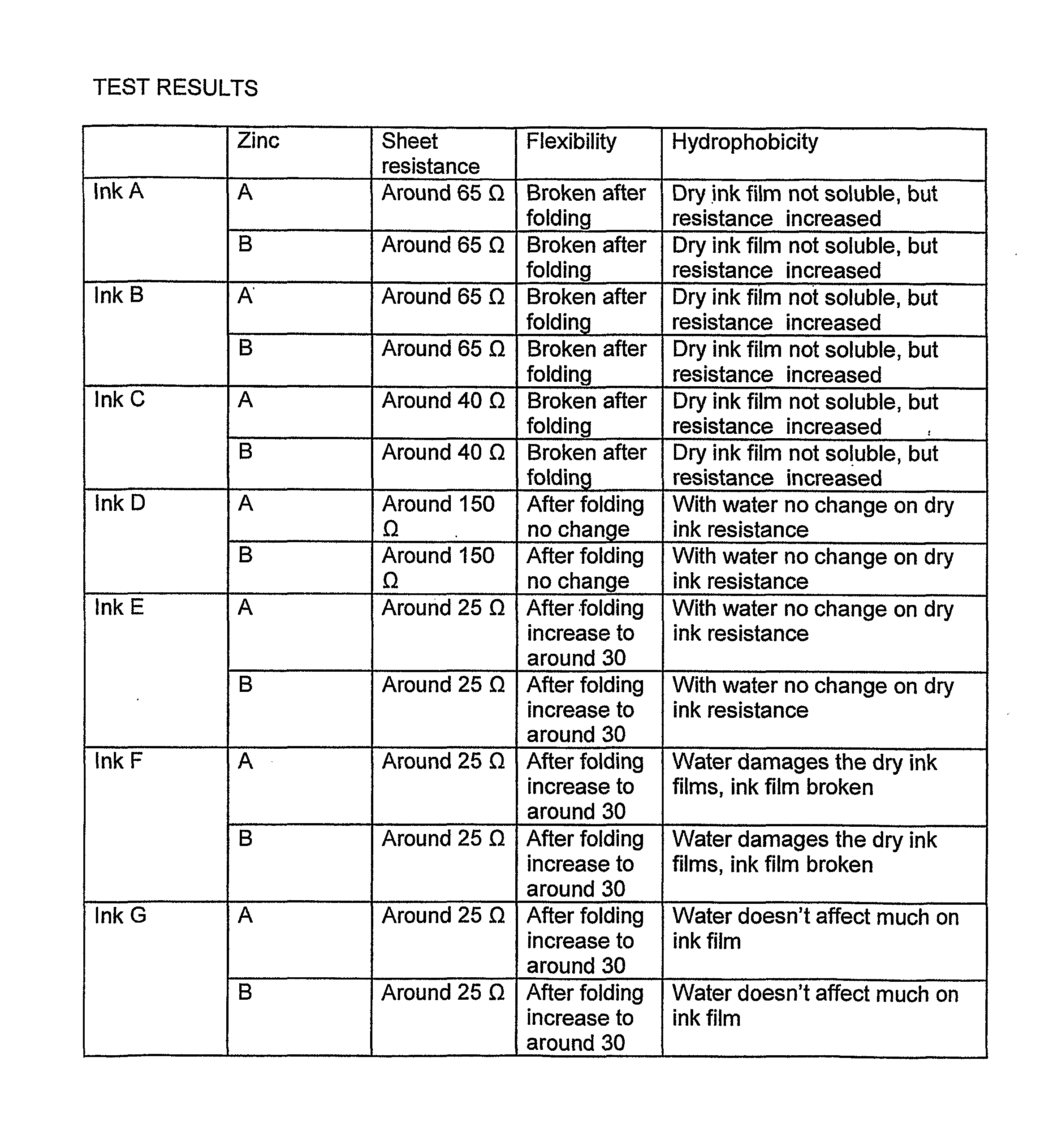
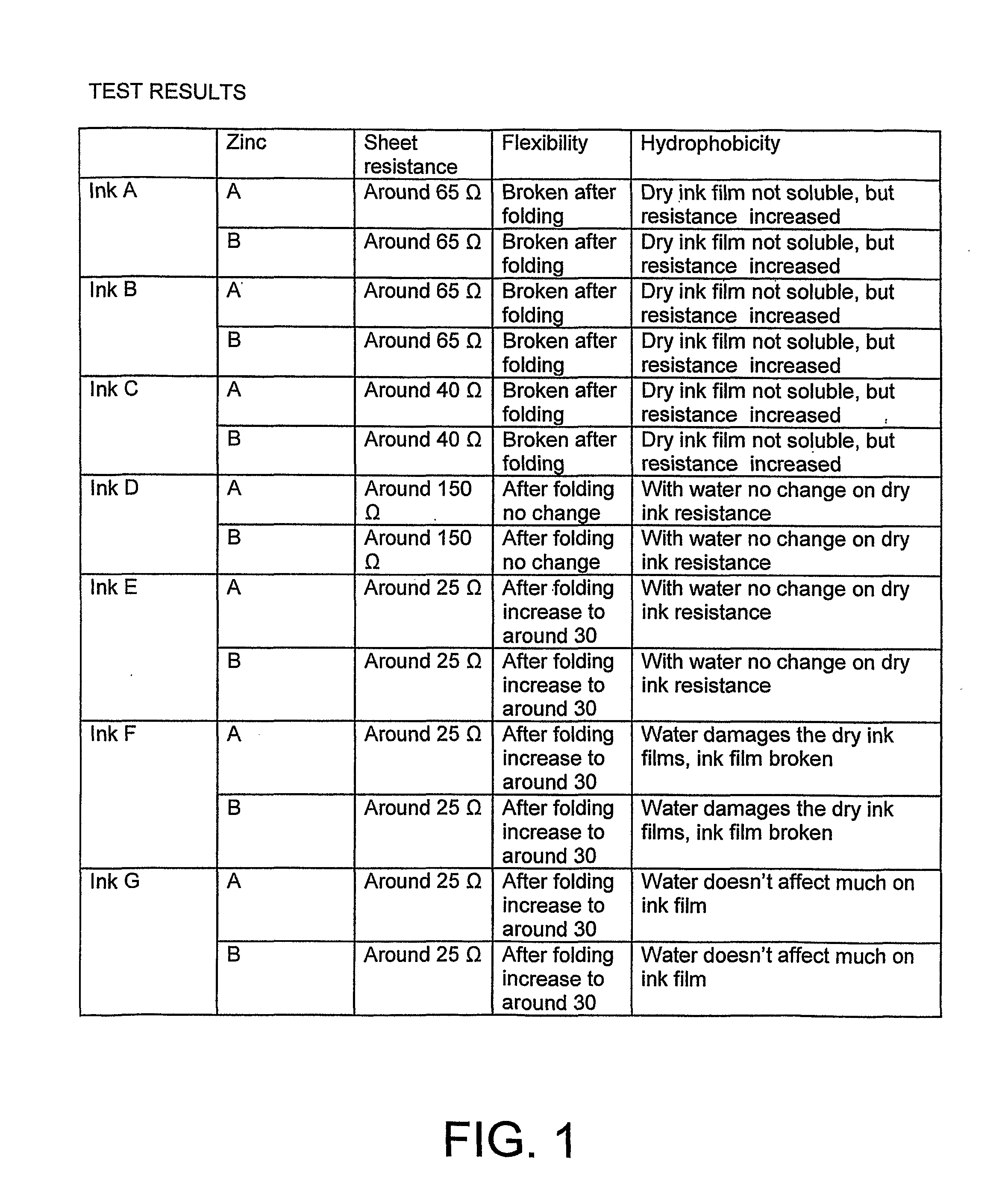
[0047]The anode material (i.e. anode ink) of the invention is prepared by adding zinc powder to conductive carbon ink and keeping stirring until homogeneous as mentioned early. The ink and zinc powder used in the test are Acheson EB 412 and EC-100.
[0048]The conductive carbon ink is stirred with a speed of e.g. 300 rpm and the zinc powder is added gradually. When the zinc powder has been added, the speed of the mixer is gradually increased up to 2000 rpm and the blend is further stirred in about 30 minutes.
[0049]The mixing in the way it is done in the invention gives a good result of anode performance, since peeling problems, conductivity problems an uneven conductivity is avoided. A proper viscosity of the anode material is needed. If the viscosity is too low, then zinc particles will deposit to the bottom. A suitable viscosity of the ink is: Brookfield 20° C. 20 RPM 20000 mPaS to 28000 mPa·S. The mixing quality can be controlled by t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com