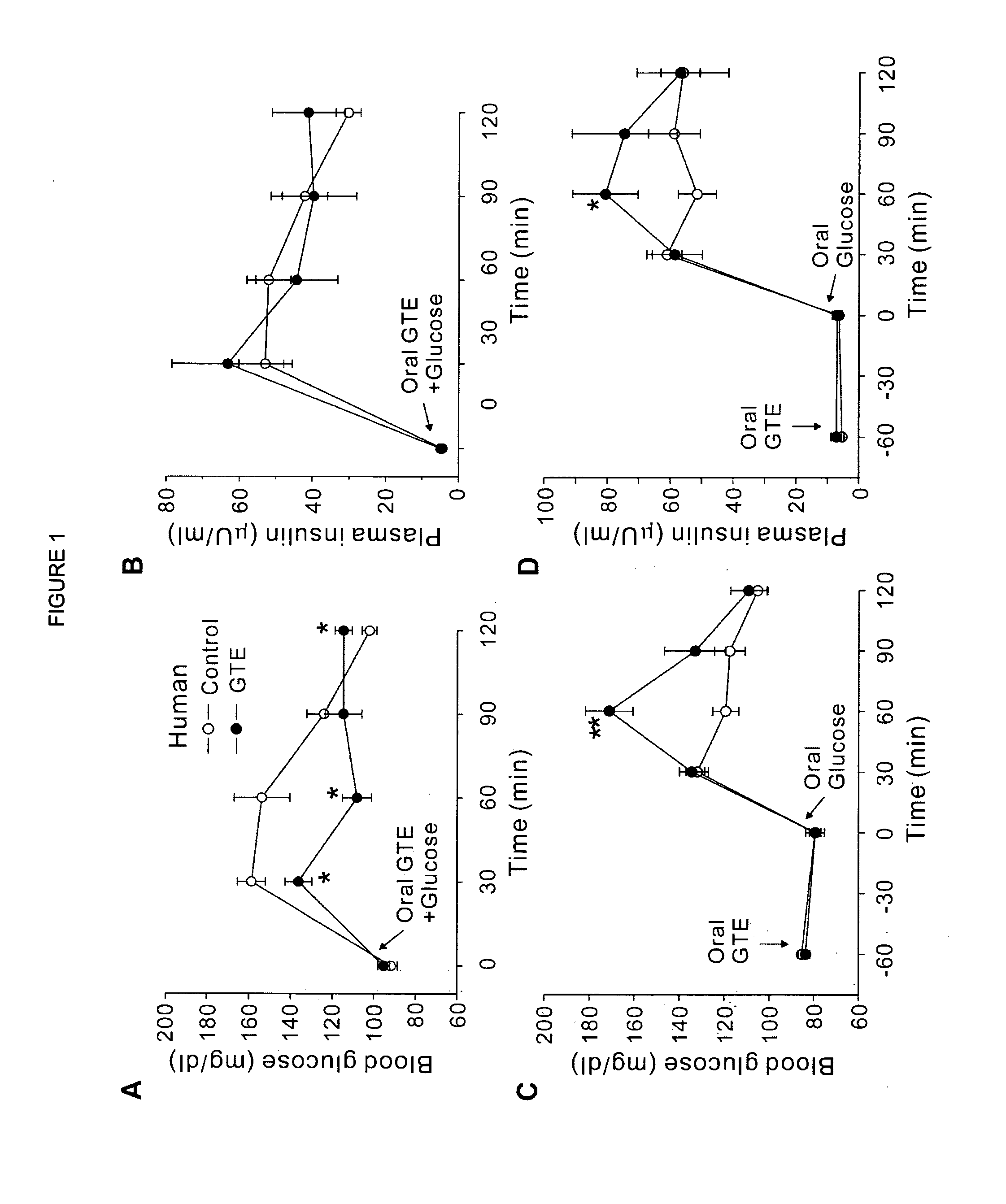
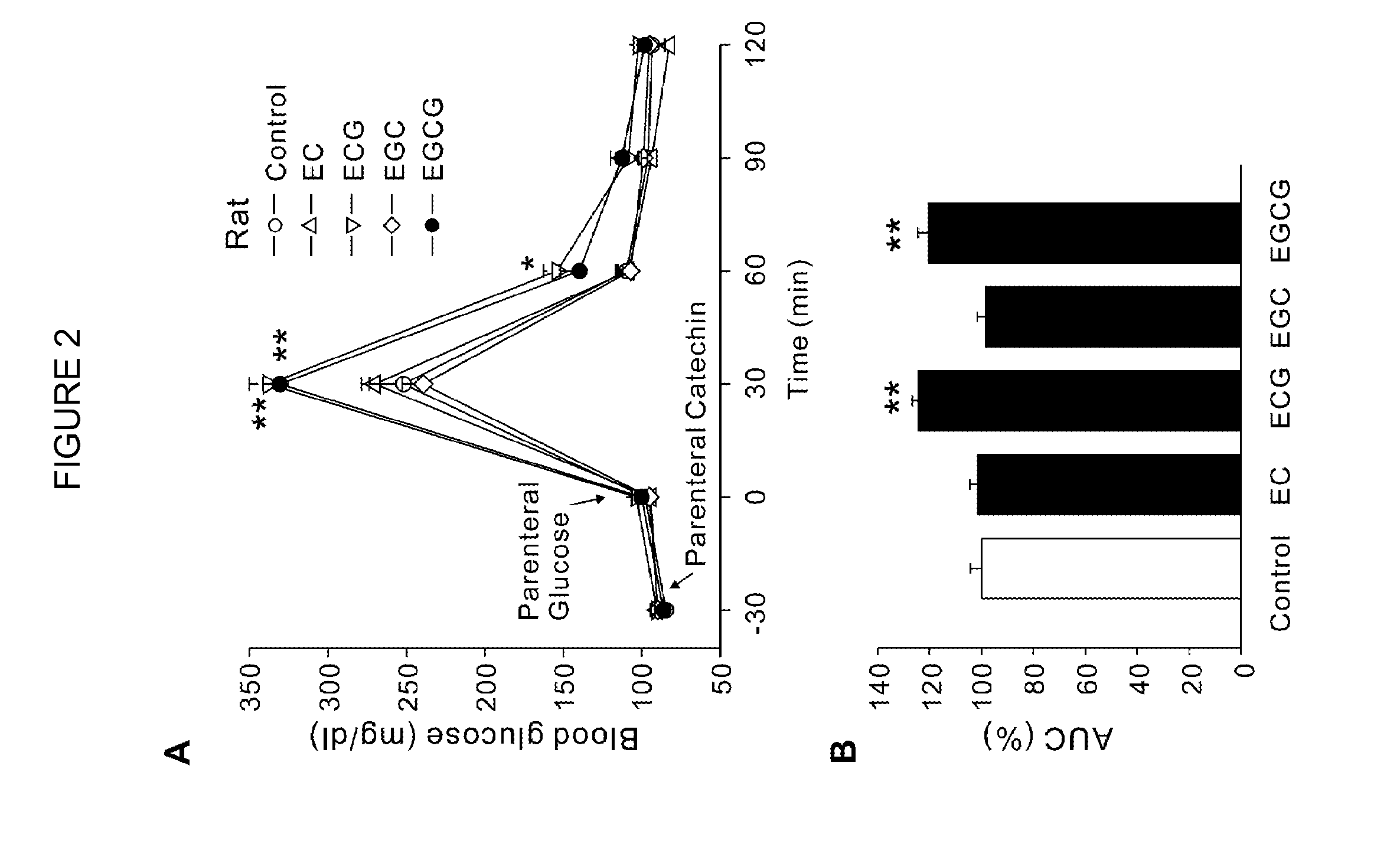
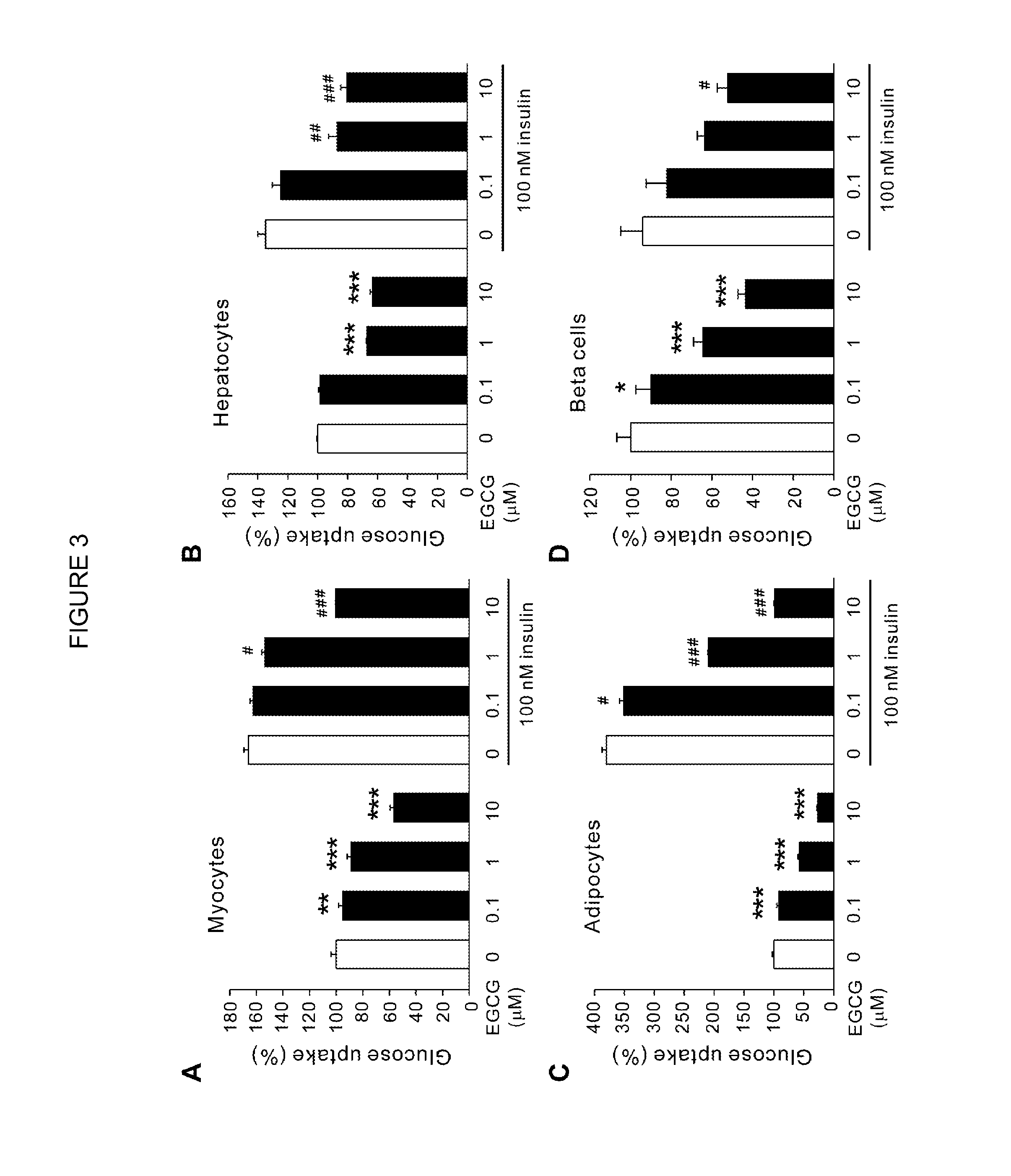
Composition for controlling increase in blood glucose
a technology of blood glucose and composition, applied in the field of composition for controlling the increase of blood glucose, can solve the problems of long postprandial hyperglycemia, use can induce some gastrointestinal side effects, and use can prolong postprandial hyperglycemia, so as to reduce glucose and cholesterol absorption, hinder 2-deoxyglucose uptake, and increase blood glucose and insulin levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Example 1.1
Materials
[0053]Dulbecco's modified Eagle's medium (DMEM), phosphate-buffered saline (PBS), fetal bovine serum (FBS) and fetal calf serum (FCS) were purchased from Gibco (Carlsbad, Calif.). RPMI-1640 medium was purchased from Welgene (Daegu, Korea). Polyethylene glycol (PEG; Novasyn TG hydroxy resin) was delivered from Novabiochem (Darmstadt, Germany) for animal study. PEG for human study was kindly gifted from Taejoon (Seoul, Korea). Green tea leaves (BOSUNG SEIJAK) were purchased from Bosung green tea Co. (Jeonnam, Korea). Epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG), epigallocatechin (EGC) and epicatechin (EC) were purchased from Sigma-Aldrich (St. Louis, Mo.). All other chemicals were obtained from Sigma-Aldrich.
example 1.2
Cell Culture
[0054]Rat L6 myoblasts were cultured in low-glucose DMEM with 10% (v / v) FBS until they reached 80% confluency. To induce differentiation, cells were further cultured in DMEM (24.9 mM glucose) containing 2% FBS for 7 days. Cell viability was assessed by the trypan blue viability test. Myogenic differentiation to myotube status was evaluated both morphologically and biochemically. Mouse 3T3-L1 preadipocytes were grown to confluence at 37° C. in 35-mm culture dishes in DMEM containing 10% FCS with no added biotin or pantothenate in incubators equilibrated with 5% CO2. At two days post-confluence (day 0), differentiation was induced with methylisobutylxanthine (0.5 mM), dexamethasone (0.5 μM) and insulin (5 μg / ml) in DMEM containing 10% FBS. On day 2, methylisobutylxanthine and dexamethasone were removed and insulin treatment was continued for 2 additional days. On day 4 and thereafter, DMEM (without insulin supplementation) plus 10% FBS was replaced every 2 days. Cells (2×1...
example 1.3
Preparation of GTE
[0055]For animal studies, 20 g of green tea leaves were added to 1,000 ml of nanopure water. After being stirred for 5 min at 80° C., the tea leaves were removed by filtration using filter paper (Advantec 2 filter paper, Hyundai micro Co., Seoul, Korea) under reduced pressure. The extract was dried by lyophilization. (A total of 3 g of GTE was harvested, in which EGCG, ECG, EGC and EC were ˜100, 53, 56 and 31 mg / g GTE, respectively). An equal amount of the GTE solution was mixed with 2 g PEG bead for 5 min at room temperature. After filtration, the supernatant was lyophilized (EGCG, ECG, EGC and EC were ˜26, 14, 42 and 25 mg / g GTE, respectively) to obtain GC-deficient GTE (GTE-GC). The results indicated that the resin pretreatment preferentially reduced GC from the GTE solution. For the human study, 30 g of green tea leaves were added to 500 ml of nanopure water. After being stirred for 3 min at 80° C., tea leaves were removed by filtration, remaining a 350 ml solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com