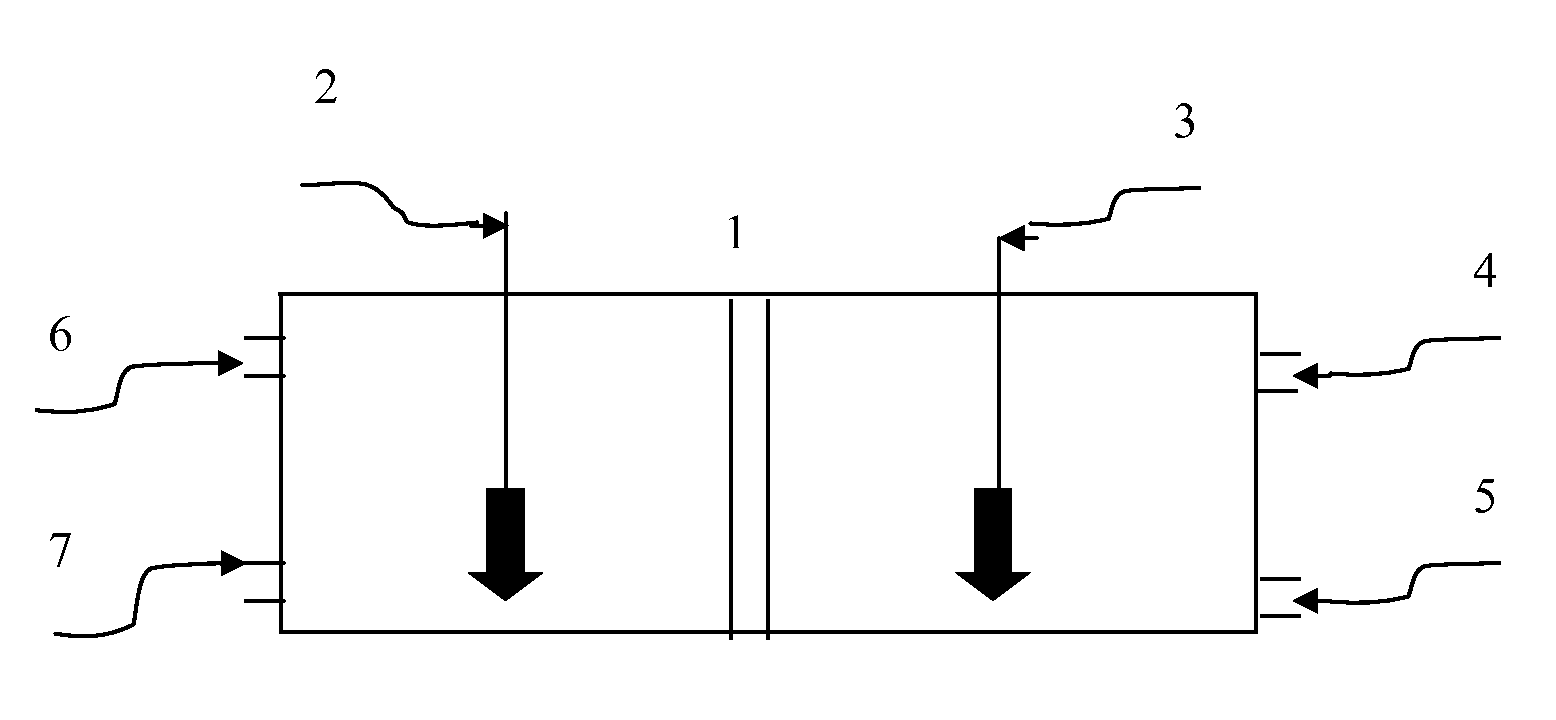
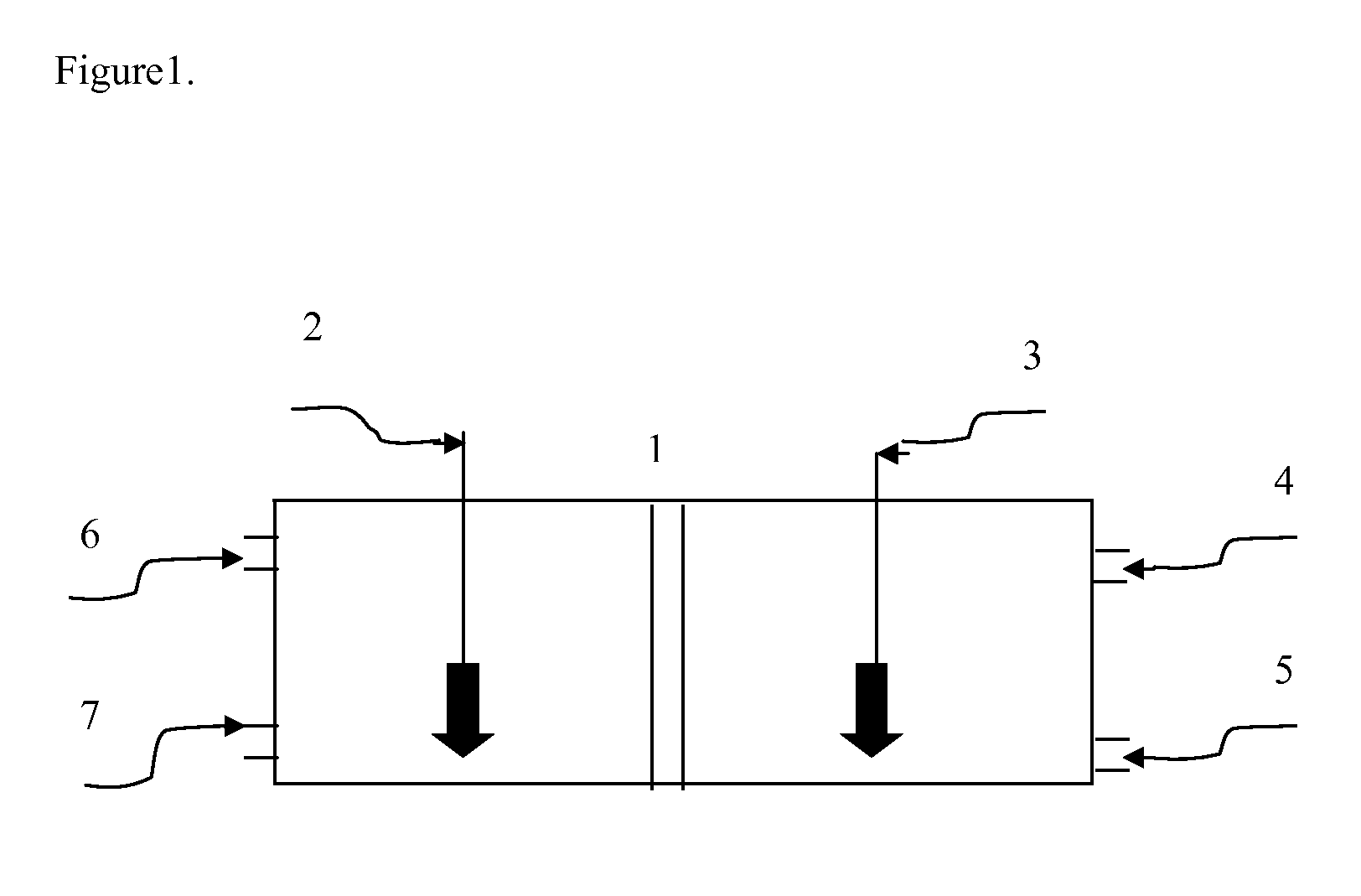
Redox membrane-based flow fuel cell
a fuel cell and membrane technology, applied in the direction of fuel cells, indirect fuel cells, electrical equipment, etc., can solve the problems of low internal electric resistance of the cell, the need for a catalyst, and the use of oxygen reduction reaction, etc., to achieve the effect of increasing the complexity of the problem, slowing down the reaction speed, and reducing the resistance of the cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 3
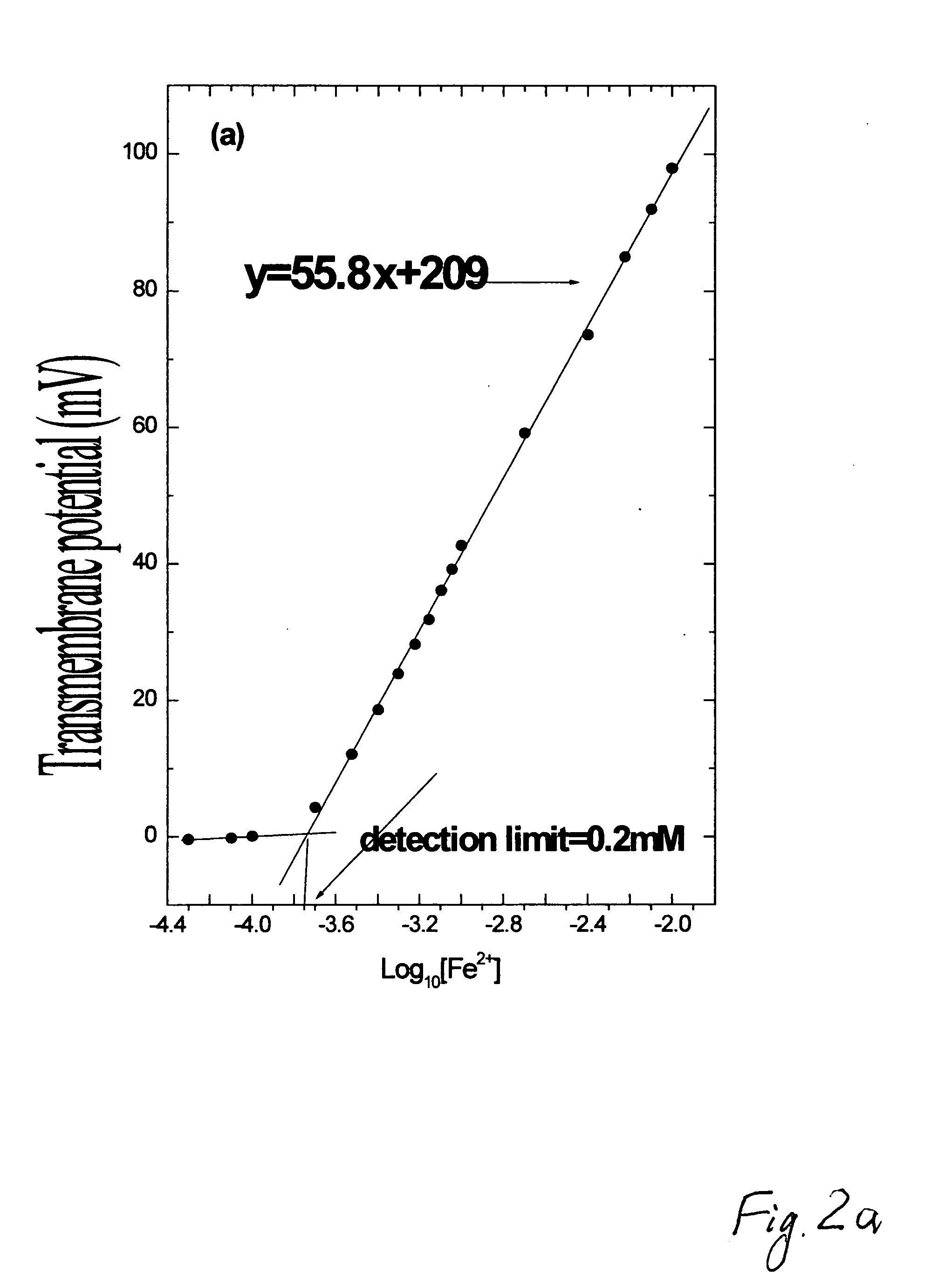
[0071]This example demonstrates that it is possible to use not only inorganic but also organic reducing agents. Ascorbic acid, NADH and NADPH, redox active dyes Neutral red, Nile blue and N-phenylanthranilic acid can be used as reducing agents reacting with PANI. The membrane had a good response to redox active substances, which normally do not have satisfactory response on Pt electrode, i.e. ascorbic acid. The slope for ascorbic acid was near 26 mV for ten times concentration changes, corresponding to the redox mechanism with two electrons transferred through the membrane from one molecule of ascorbic acid. The lower concentration limit when these substances start changing transmembrane potential is near 0.2 mM in 0.01M phosphate buffer, pH 6.4.
example 4
[0072]Combination of the membrane together with many different redox agents, which can easily react with PANI and have different redox potentials can be used as a source of electric energy. This example demonstrates that using strong oxidizing agents at higher concentrations leads to higher and practically important transmembrane voltage. PANI can electro-catalyze the reduction of O2 in sulfuric acid in fuel cell operations with H2O2 as the product:
LEB+2A−+2H++O2→ES+H2O2 (1)
Here LEB and ES refer to leucoemeraldine base and emeraldine salt, respectively; A− is the anion from solution, which is then incorporated into ES as the counter ion. Freshly made polyaniline in acidic solutions in contacts with air can easily change its color from green to dark blue and then to black even without electrodes because of redox processes. Mechanism of possible continuous H2O2 formation and PANI regeneration using reducing potential is presented in the Scheme 1.
[0073]Thermodynamic electrode potentia...
example 5
[0075]This example demonstrates that transmembrane potential can be additionally influenced 425 by changes of chloride anion concentration. Addition of potassium chlorides into the reducing solution makes increases the transmembrane voltage and makes it even more negative. Addition of KCl into the opposite solution makes decreases the value of transmembrane voltage.
[0076]The transmembrane electric potential difference across the doped PANI membrane is a mixed potential due to both electron transport in redox processes and simultaneous Cl− ion transport. When the first reversible pare of redox agents is present in one of the solutions and the second in another, transmembrane voltage is described by the equation
V=ΔE0-2.3RTnFLogred1+αCl1-ox1-βCl1-+2.3RTnFLogred2+αCl2-ox2-βCl2-
Here red, ox and Cl− are activity of corresponding species; subscripts 1 and 2 correspond to the two solutions separated by the membrane; and α and β characterize membrane selectivity for pares red / Cl− and ox / Cl−....
PUM
| Property | Measurement | Unit |
|---|---|---|
| currents | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com