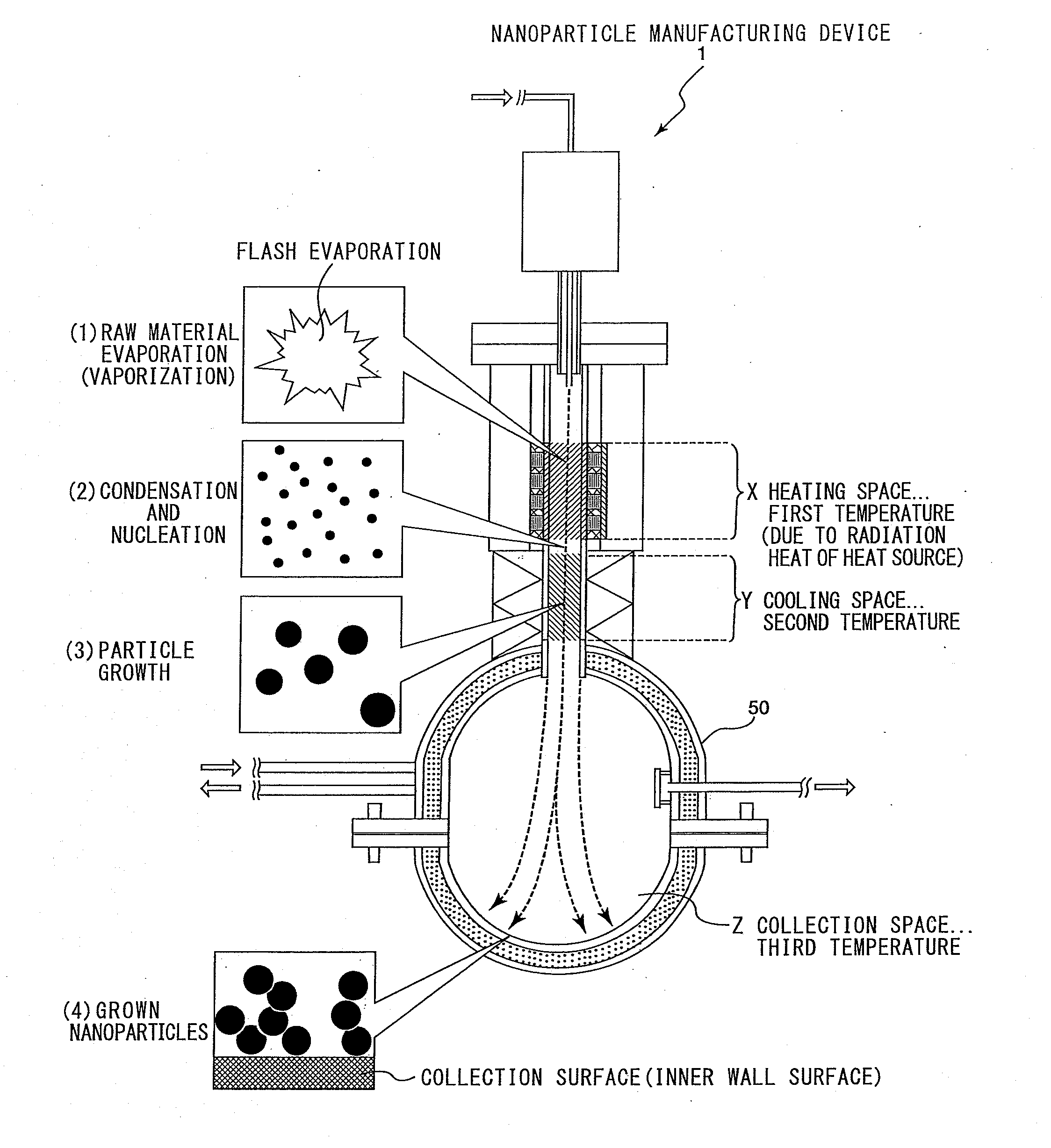
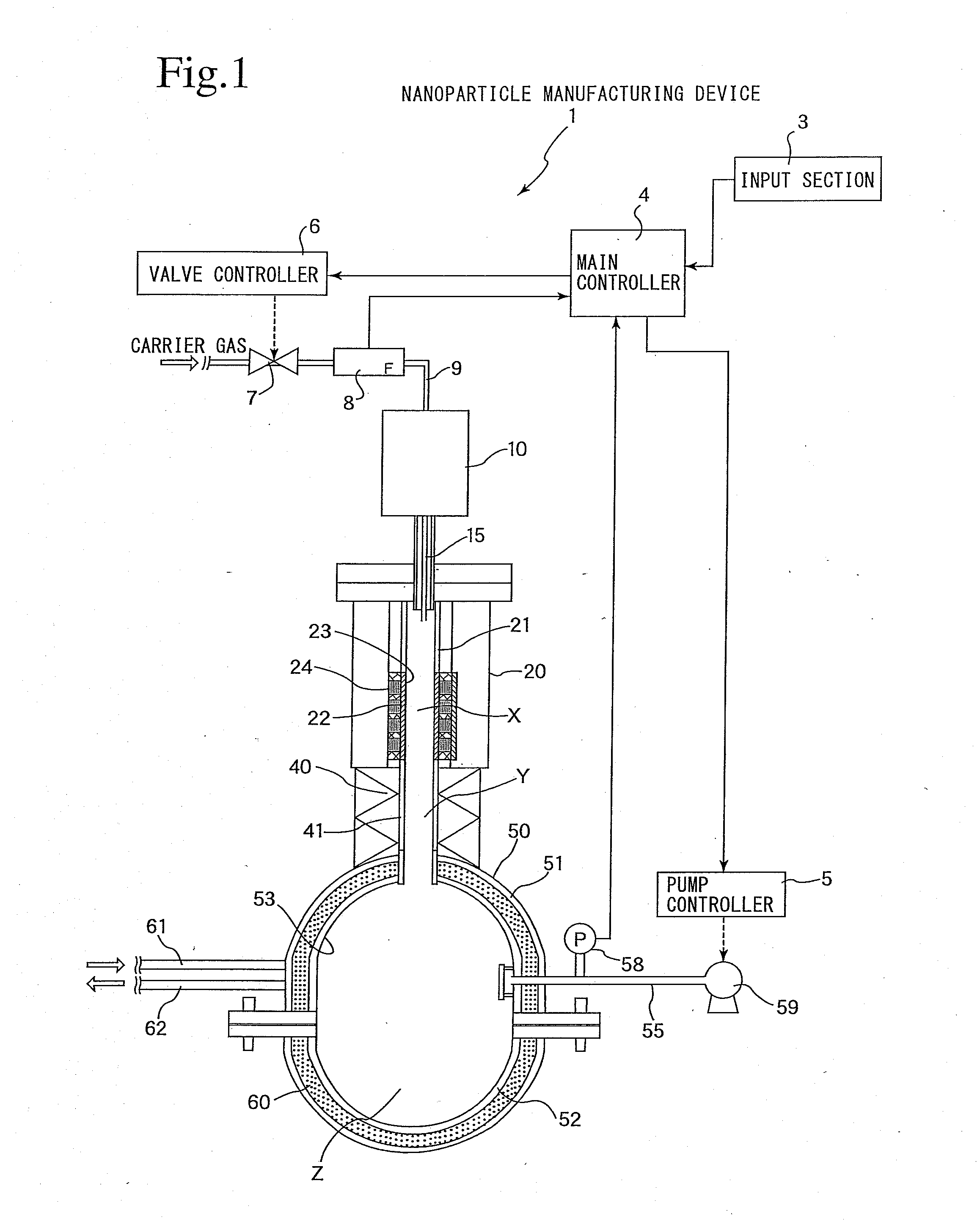
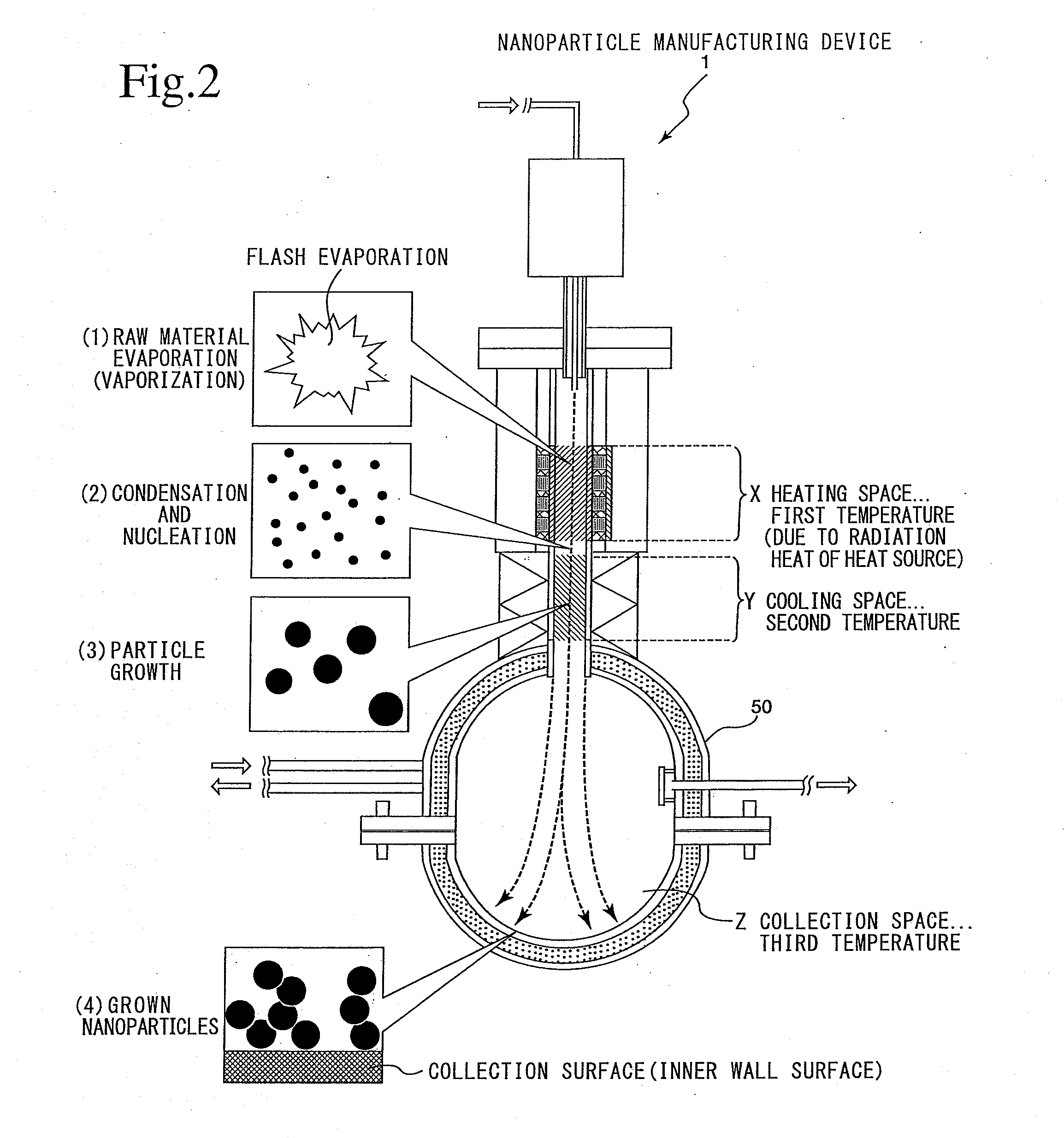
Nanoparticle manufacturing device and nanoparticle manufacturing method and method of manufacturing nanoparticle-dispersed liquid alkali metal
a technology of nanoparticles and manufacturing methods, applied in the direction of transportation and packaging, energy-based chemical/physical/physical-chemical processes, chemical/physical/physical-chemical processes, etc., can solve the problems of high construction cost, high construction cost, and severe chemical reactions, so as to improve the suppression of thermal conductivity and chemical activity, good nanoparticle dispersibility, and stable maintenance of fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
for the Liquid Alkali Metal
[0111]As Embodiment 1 for the liquid alkali metal, a description will be given of the case where the liquid alkali metal is liquid sodium and the nanoparticles are made of titanium.
[0112]In this embodiment, the inventors adopted a method in which a slurry flows only by a propulsive force by ultrasonic waves and the slurry flows efficiently into the ultrasonic irradiation region (the forward end portion of the homogenizer tip 102; a tip 102 having a diameter of 3 mm was adopted here), and they ascertained that the titanium nanoparticles tend to decrease in size.
[0113]Concretely, the particle diameters were measured by use of the particle diameter measuring device LB-550 made by HORIBA, Ltd. and it was ascertained that particle diameters as median size (D50: based on the number of particles), which were several micrometers only by stirring before the ultrasonic irradiation processing, decreased to 109 nm after a lapse of 60 minutes.
[0114]In order to stably d...
embodiment 2
for the Liquid Alkali Metal
[0119]A dispersion test in liquid sodium was carried out using a sample prepared by the manufacturing method of the present invention which is such that the liquid alkali metal of Embodiment 1 is liquid sodium and the nanoparticles are made of titanium.
[0120]The dispersibility of titanium nanoparticles was evaluated by a method which involves causing a sample subjected to ultrasonic irradiation processing to stand. Concretely, a sample irradiated with ultrasonic waves was caused to stand, with the temperature of liquid sodium maintained at a liquid temperature of 350° C., and the titanium concentration remaining in a supernatant liquid of the sample was measured by use of ICP (inductively coupled plasma) (the high-frequency plasma emission spectrometer ICPS-8100 made by Shimazu Corporation) immediately after the ultrasonic irradiation processing, after a lapse of 24 hours and after a lapse of 96 hours. FIG. 11 is a graph showing the results of a standing t...
embodiment 3
for the Liquid Alkali Metal
[0122]Next, an ultrasonic irradiation test was conducted in a case where the resistance time of ultrasonic waves in liquid sodium is changed. FIG. 12 is a graph showing the results of the dispersibility test in which the dispersibility of nanoparticles of a nanoparticle-dispersed liquid sodium produced by the manufacturing method in an embodiment of the present invention changes as a result of changes in the residence time of ultrasonic waves.
[0123]It was ascertained that when the resistance time of ultrasonic waves is changed in liquid sodium, the particles aggregate and the dispersibility becomes worsen when the residence time is too long. In order to improve the dispersibility, it is preferred that the residence time be 5 to 20 seconds when the ultrasonic output density is 460 W / cm2. On the other hand, the output density may take on small values of the order of several to several tens of watts per cm2. In this case, because the surface of the ultrasonic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com