Medicament for the Treatment of Pain and Inflammation
a technology applied in the field of medicine for the relief of pain and inflammation, can solve the problems of difficult treatment, poor response to standard pain treatment, and neuropathic pain, and achieve the effects of convenient and safe treatment, minimal risk of undesirable side effects, and minimal risk of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
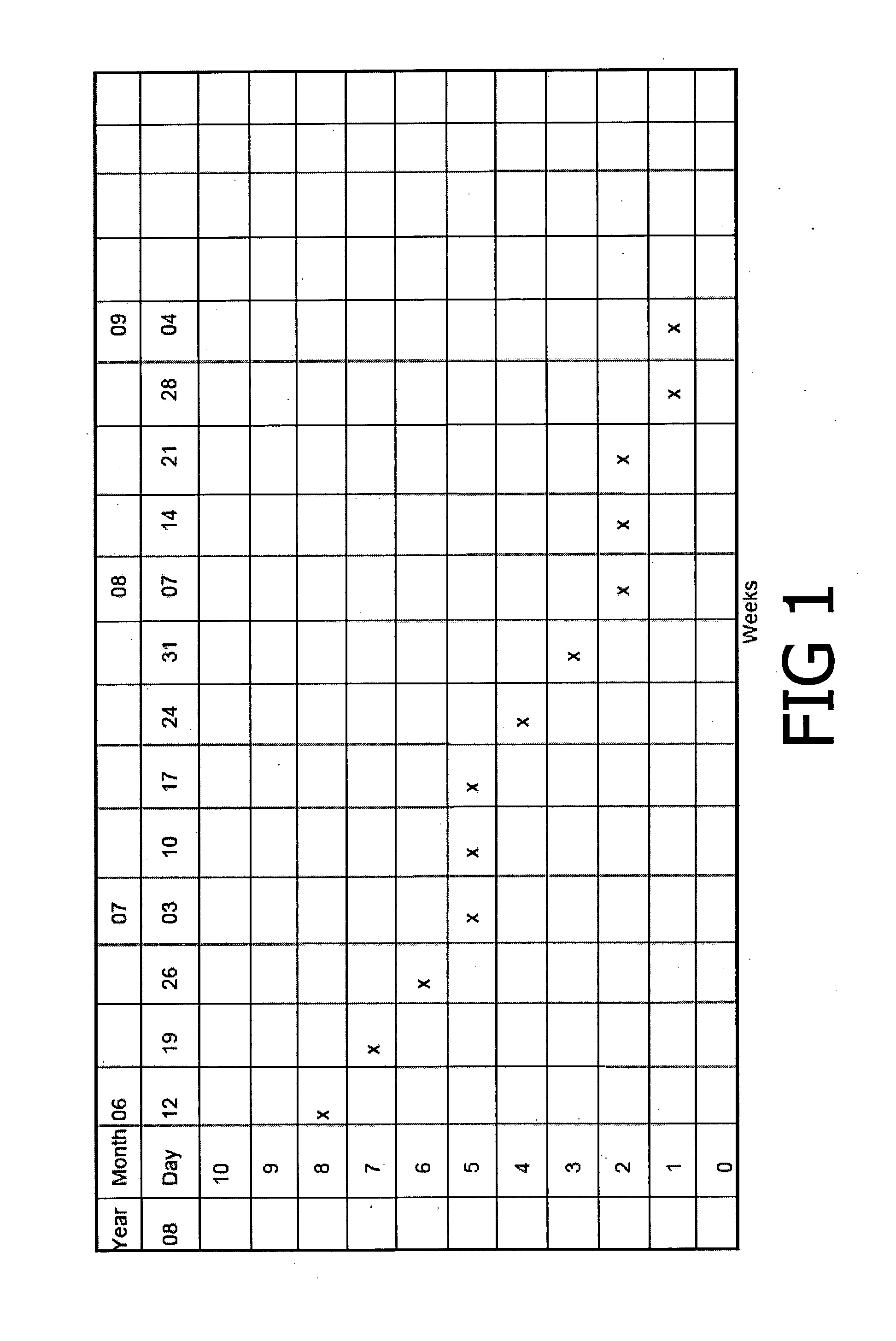
[0022]Patient 1—History: MM, age 46, female, elite masters middle distance runner with 2-month history of right inferior heel pain treated initially with subcutaneous prolotherapy, complicated by infection, complaining of inability to run, difficulty walking, and pain at rest. Past history of bilateral peripatellar pain treated with failed surgical decompression, Achillodynia.
[0023]The patient applied the cream by massaging the cream into the painful area twice a day and after any activity, with the massaging followed by the application of heat where possible. This procedure was followed until all pain and tenderness had disappeared from the area. The treatment was continued for three months, and an assessment of pain was recorded every seven days, as shown in FIG. 1. Over this period, the pain reduced from a severe pain to very little pain, with the pattern of pain reduction being a period of steady decrease of pain followed by a plateau, then a further steady decrease followed by ...
example 2
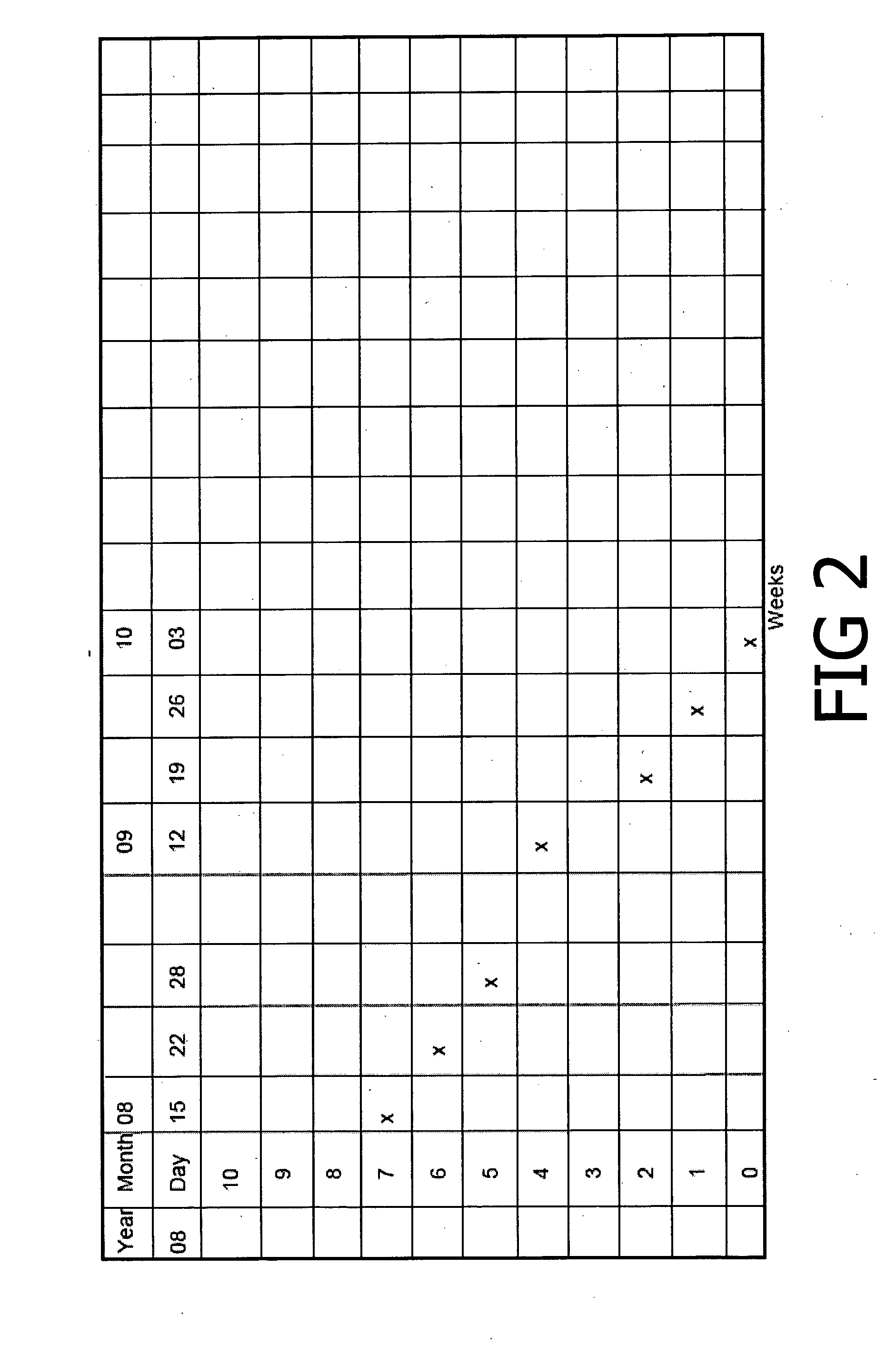
[0024]Patient 2—History: JF, age 24, female, fitness trainer with 8-month history of left inferior heel pain, complaining of inability to run, difficulty walking, affecting work. Has prior treatment with physiotherapy and podiatry without effect. Past history of bilateral Osgood Schlatter Disease, right medial shin splints and peripatellar pain.
[0025]The patient applied the cream by massaging the cream into the painful area twice a day and after any activity, with the massaging followed by the application of heat where possible. This procedure was followed until all pain and tenderness had disappeared from the area. The treatment was continued for two months:—as shown in FIG. 2, over this period the pain steadily diminished from severe pain to no pain. This patient is still using the cream, and continues to run with no pain.
[0026]The patients from Examples 1 and 2 were monitored for serum vitamin D levels at the end of treatment; both showed normal levels.
example 3
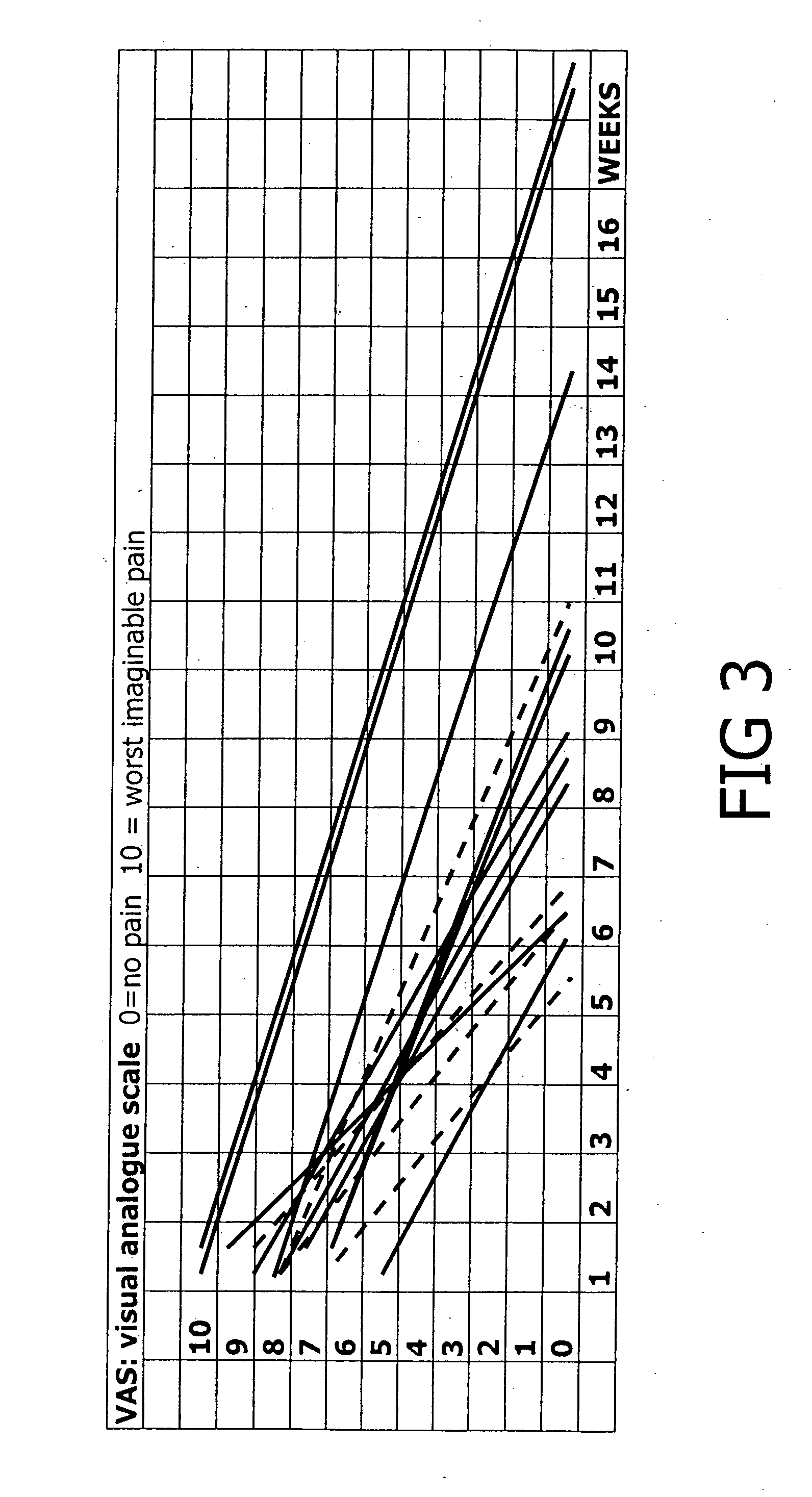
[0027]A total of 14 patients, all suffering from recalcitrant inferior heel pain (plantar fasciitis) were studied over a period of several months. The patients consisted of three males with a mean age of 56 (range 50 to 60) and 11 females with a mean age of 44 (range 23 to 61).
[0028]Inferior heel pain (plantar fasciitis) is a difficult to treat condition, causing high levels of pain and disability for up to four years in one prospective study of 100 patients (BMJ Clinical Evidence Concise June 2006)
[0029]The underlying cause of inferior heel pain is postulated to be due to chronic neurogenic inflammation of the distal Tibial nerves branches known as the medial cutaneous calcaneal nerves.
[0030]All patients in this study had received prior treatment including physiotherapy, cortison injections and podiatry with taping and orthotics without benefit.
[0031]The patients were treated with a cream consisting of a known cream base of a type suitable for transdermal applications of medication...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
| fat soluble | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com