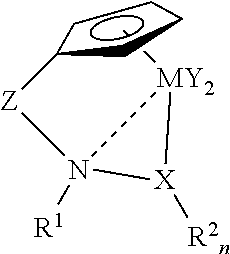
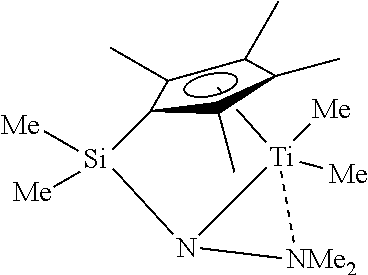
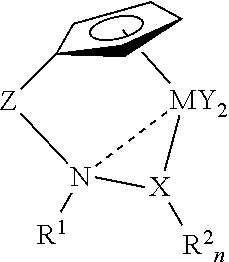
Catalysts for olefin polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 34
Synthesis of Complex 1
[0028]
[0029]Following the method of Bernardi et al. (J. Org. Chem. 64 (1999) 641, N-methylhydroxylamine hydrochloride is combined with 1.1 eq. of 2,4,6-trimethylpyridine in pentane at room temperature for 12 h, followed by treatment with 1 eq. of 1-(trimethylsilyl)imidazole with stirring for 9 h. Filtration, concentration, and distillation should provide N-methyl-O-(trimethylsilyl)hydroxylamine.
[0030]n-Butyllithium (1.1 eq.) is added to N-methyl-O-(trimethylsilyl)hydroxylamine in THF at −78° C. to generate the corresponding amido anion. Dimethylfulvene (1 eq.) is slowly added at −78° C. The stirred mixture is allowed to warm to room temperature for 2 h, and it is then re-cooled to −78° C. Titanium tetrachloride (1 eq.) in dry toluene is slowly added to the cyclopentadienyl anion, and the mixture is warmed to room temperature and stirred overnight. The mixture is refluxed to eliminate trimethylsilyl chloride. After extractive workup, the titanium complex is isol...
example 35
Synthesis of Complex 4
[0031]
[0032]Dichlorodimethylsilane is reacted with one equivalent of sodium cyclopentadienide in ether to give (Cp)Me2SiCl. Separately, n-butyllithium (1.1 eq.) is added to N-methyl-O-(trimethylsilyl)hydroxylamine (prepared as described in Example 34) in THF at −78° C. to generate the corresponding amido anion. The amido anion and an equivalent of the silyl chloride are combined at −78° C., followed by stirring the mixture at room temperature overnight. This should provide, after workup, the desired ligand precursor, (Cp)Me2SiNMe-OTMS.
[0033]Deprotonation with n-butyllithium to give a cyclopentadienide, followed by reaction in toluene with titanium tetrachloride and refluxing should eliminate chlorotrimethylsilane and give a titanium dichloride complex, which can be reacted with two equivalents of methyllithium to give 4.
example 36
Synthesis of Complex 5
[0034]
[0035]N,N′-dimethylhydrazine is converted to its monolithium salt by reaction with 1.1 eq. of n-butyllithium in THF at −78° C. The mixture warms to room temperature for 2 h, and is then chilled to −78° C. The mixture is combined with one eq. of CpMe2SiCl at −78° C. and stirred overnight at room temperature. After workup, the expected product is the desired ligand precursor, (Cp)Me2-SiN(Me)-N(Me)TMS. Repeating the remaining steps as in Example 35 should provide complex 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Substance count | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com