Novel use of ec-sod and method for preparing thereof
a technology of ecsod and ecsod protein, which is applied in the field of new ecsod protein use, can solve the problems of insufficient examination of the relationship between sods, angiogenesis, and removal of reactive oxygen species, and achieve the effects of inhibiting angiogenesis, preventing or inhibiting the over-differentiation of th2 cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Recombinant EC-SOD Protein Having Biological Activity
Cloning of Fusion EC-SOD Gene for Expression of Recombinant EC-SOD Protein
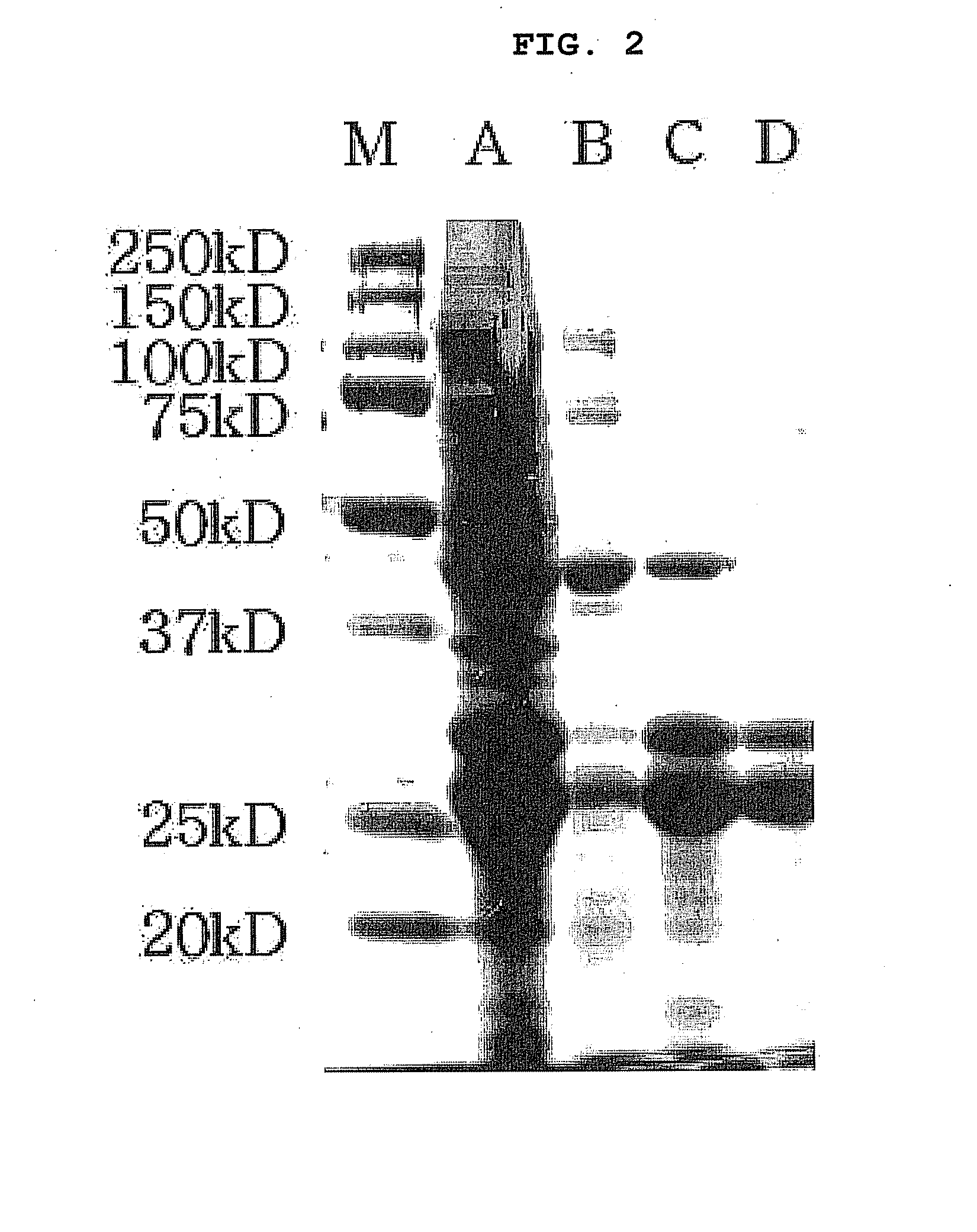
[0097]In order to prepare a recombinant EC-SOD protein having biological activity, recombinant EC-SOD, consisting of 209 amino acids and containing deletion of the signal peptide at the N-terminal end of human EC-SOD and deletion of 13 amino acids at the C-terminal end, was cloned in the following manner. The base sequence and amino acid sequence of the recombinant EC-SOD are set forth in SEQ ID NO: 2 and SEQ ID NO: 1, respectively, and the base sequence and amino acid sequence of the human EC-SOD are set forth in SEQ ID NO: 4 and SEQ ID NO: 3, respectively.
[0098]A pUC 18-hEC-SOD vector (provided by Professor Marklund, Clinical Chemistry, Sweden) containing human EC-SOD cDNA (SEQ ID NO: 4), asa template, was amplified by PCR using the following primers, thus preparing human EC-SOD cDNA:
Sense primer(SEQ ID NO: 9)5′-tagattctggacgggcgagga-3′Anti...
example 2
Angiogenesis Inhibitory Effect of Natural EC-SOD Protein
[0115] Morphological Comparison of EC-SOD-Expressing Mice with Wild-Type Mice
[0116]The back skin of each of the mouse EC-SOD-overexpressing mice, prepared in Test Example 1, and wild-type BDF1 mice, was irradiated four times with UVB at a dose of 2 kJ / m2 at 24-hr intervals. Specifically, the back skin of each mouse was shaved at 2 days before UVB irradiation, and twenty-four 8-week-old mice were irradiated with UVB at a dose of 200 mJ / cm2 using 6 UVB lamps (FS24T12 / UVB / HO, 290-320 nm, Voltare, Fairfield, Conn., USA) having the highest energy at 305 nm in a wavelength range from 280 μm to 340 nm. During the UVB irradiation, the height of the lamps was controlled such that the surface of the back skin of each of the anesthetized mice was irradiated with a dose of 0.3 mW / cm2. The mice of each group (n=6) were irradiated four times with UVB at 24-hr intervals, and at 1 hour after the 4th UVB irradiation, the mice were sacrificed fo...
example 3
Angiogenesis Inhibitory Effect of Recombinant EC-SOD Protein
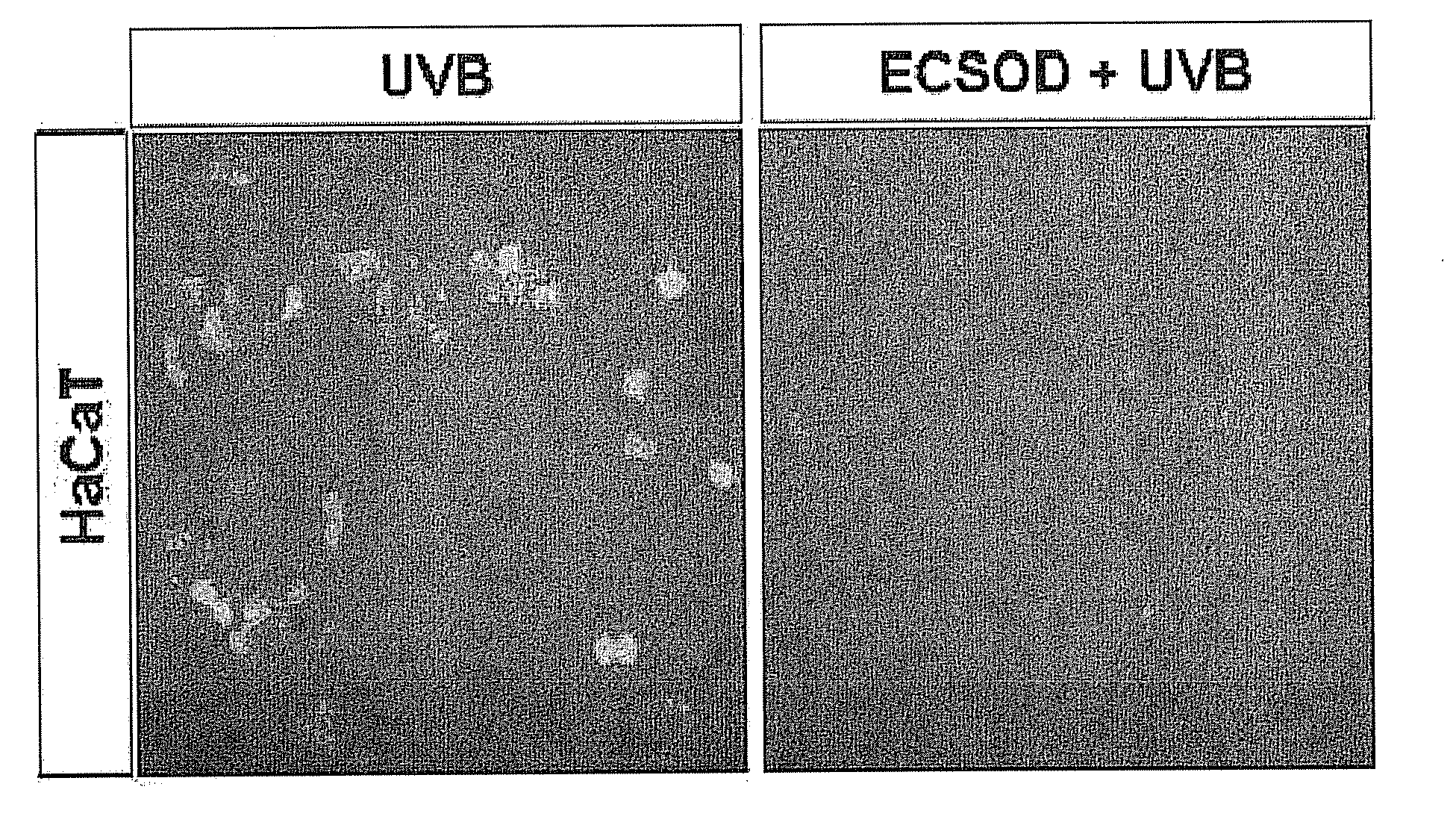
[0129]Whether the recombinant EC-SOD protein, separated and purified in Example 1, has the effect of inhibiting angiogenesis was examined. Keratinocyte HaCaT cells were plated on 18-mm cover slips at a concentration of 1×104 cells / cover slip. The cells were cultured in DME medium (Dulbecco's minimum essential medium, GIBCO), containing 10% fetal bovine serum (FBS, GIBCO) and 100 units penicillin and 100 g streptomycin / ml of medium, in 5% CO2 at 37° C. When the adhered cells reached a confluence of 30-40%, the fetal bovine serum was removed, and then cultured for 24 hours. When the cells reached a confluence of 50-60%, the cells were pretreated with 10 μg of EC-SOD. After the cells were treated with the purified recombinant EC-SOD protein for 30 minutes, the cells were treated with UV light at a dose of 100 J, and after 24 hours, the skin keratinocyte cells were fixed with paraformaldehyde and immunostained. The fixed cells ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com