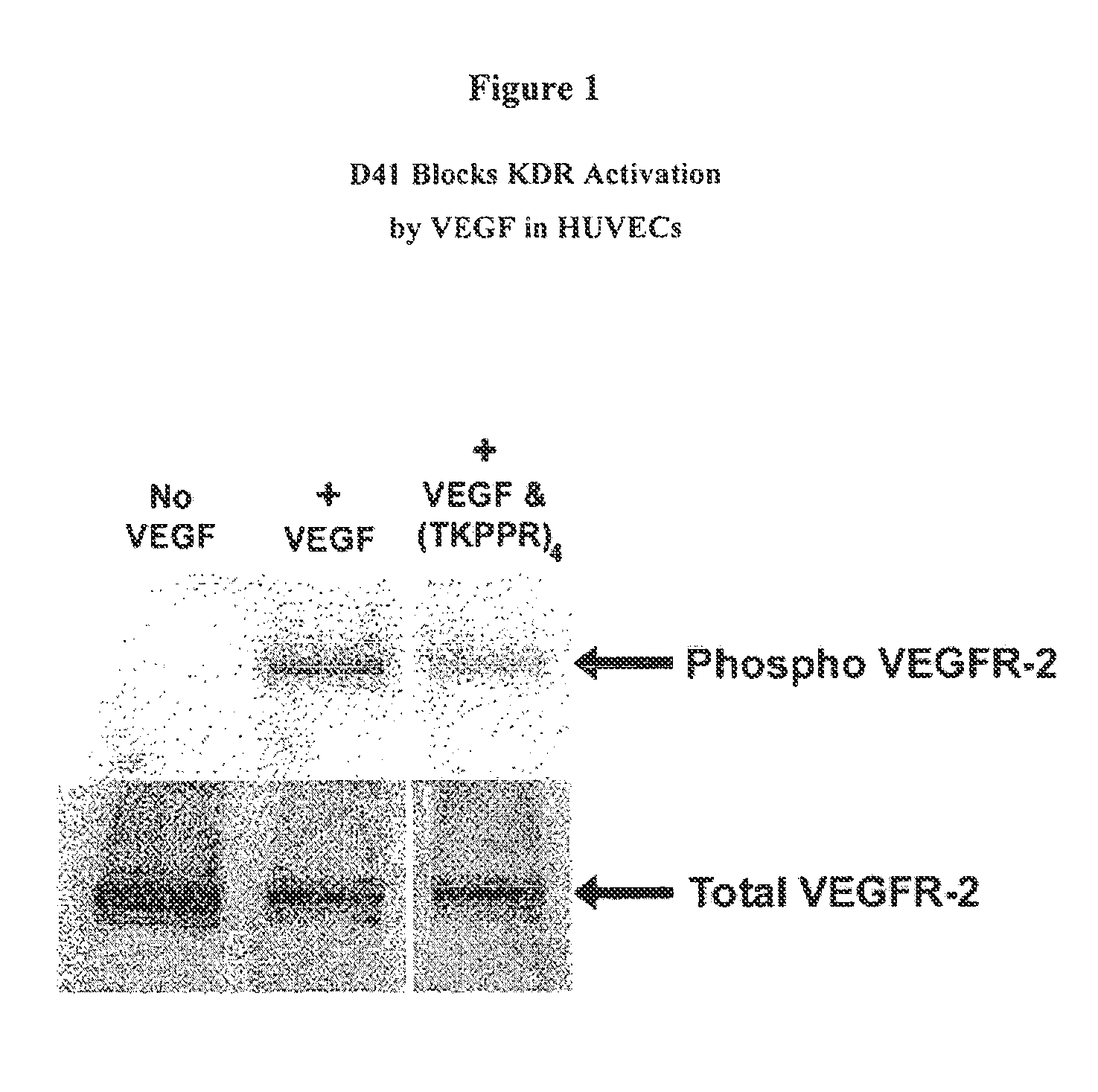
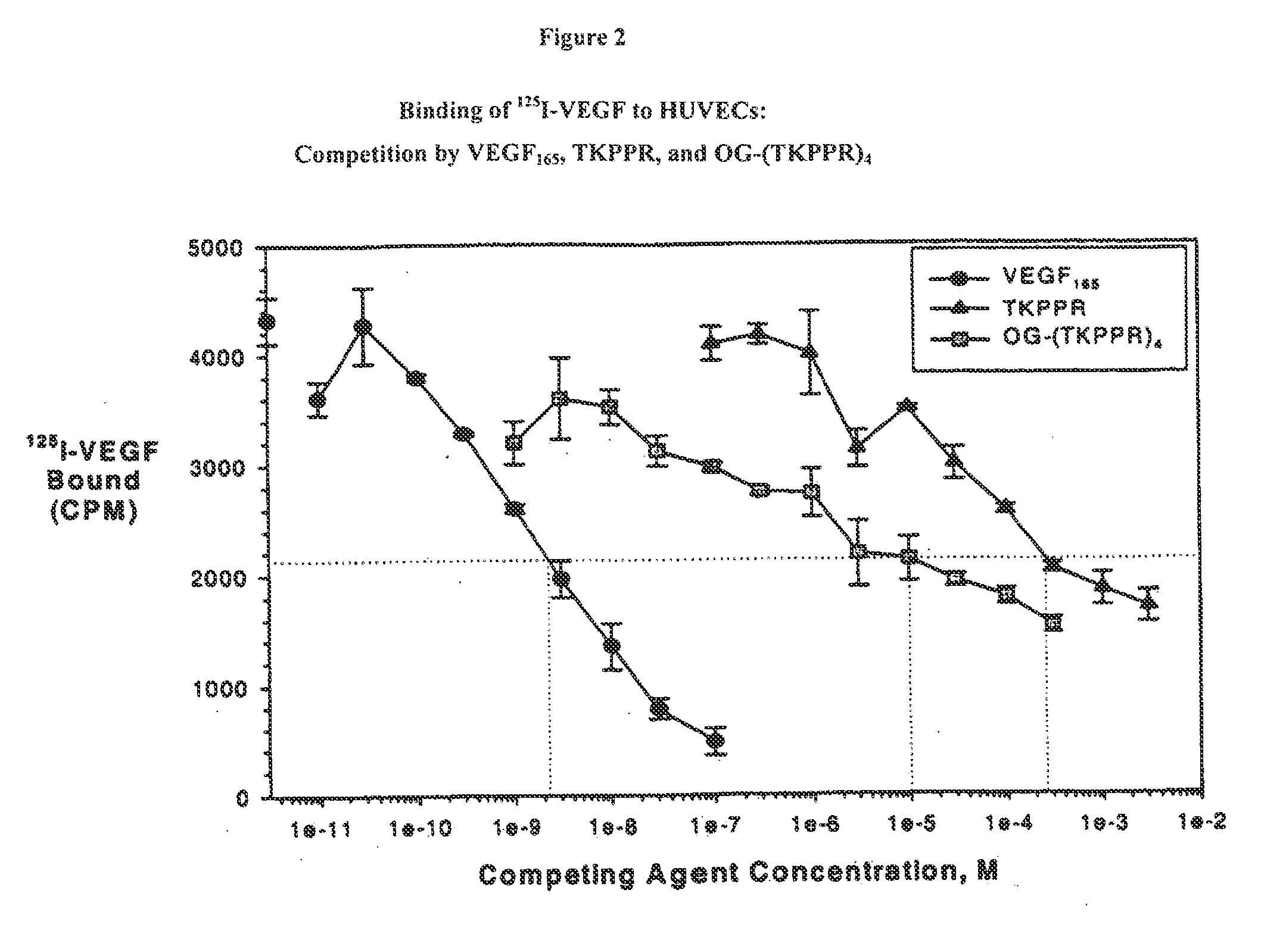
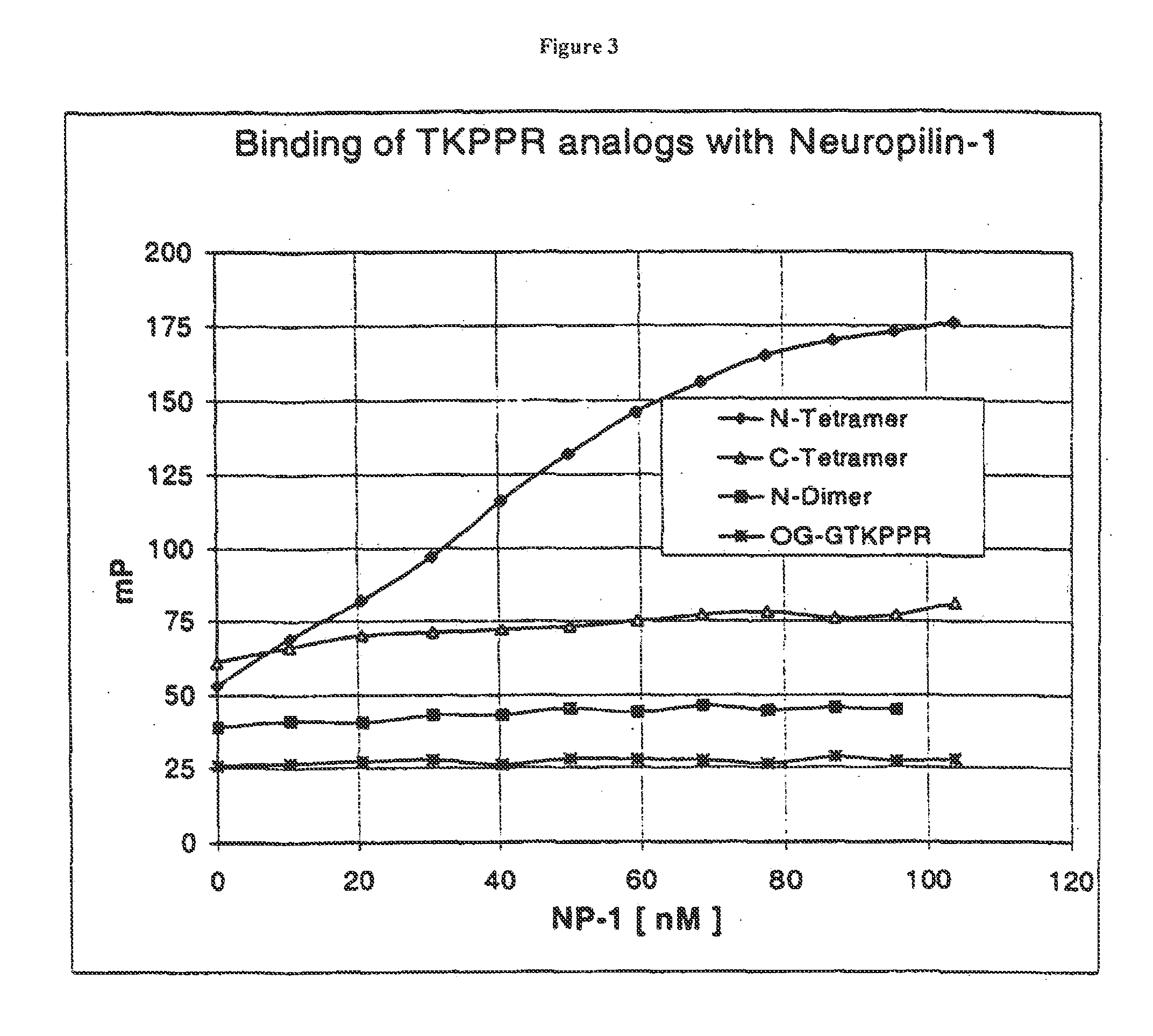
Compounds for Targeting Endothelial Cells, Compositions Containing the Same and Methods for Their Use
a technology of endothelial cells and compounds, which is applied in the field of compounds for targeting endothelial cells, compositions containing the same and methods for their use, can solve the problems of limited use of echocardiography for the diagnosis of cardiovascular diseases, undetected accumulation of tuftsin labeled with a radionuclide metal in non-target tissues, etc., and achieves the effect of superior binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of TKPPR-OH
[0413]
A. Preparation of Pro-Pro-Arg(NO2)Obzl
[0414]
[0415]To a solution of Boc-Pro-Pro-OH (commercially available) (3.2 g, 10.25 mmol) in methylene chloride (100 mL) was added Arg(NO2)Obzl.PTSA salt (commercially available) (6.54 g, 10 mmol) and the mixture was stirred for 5 min. This mixture was cooled to 5° C. and HATU ([0-(7-azabenzotriazol-1-yl)1,1,3,3,-tetramethyluronium hexafluorophosphate], (commercially available), (3.9 g, 10.25 mmol) was added in one lot followed by diisopropylethylamine (6.5 g, 50 mmol). After stirring the reaction mixture for 12 h at room temperature, the solvents were removed in vacuo, the residue dissolved in ethyl acetate and washed with saturated sodium bicarbonate, sodium bisulphate and finally with water. The organic layer was dried and solvent removed to afford the coupled product. This was purified by column chromatography over silica gel using 5% methanol in ethyl acetate as the eluent. Fractions containing the pure material ...
example 2
A. Cell Culture
[0457]Human aortic endothelial cells (HAEC) from Biowhittaker were grown as monolayers in EGM-MV medium from Biowhittaker according to the supplier's instructions.
[0458]Briefly, a frozen cryovial of cells (500,000 cells in about 1 mL) was thawed for 2-3 minutes in a 37° C. water bath and cells were seeded into a T-75 flask coated with collagen I (commercially available) containing 15 mL EGM-MV of medium pre-equilibrated with 5% CO2 atmosphere. Cells were incubated in a standard tissue culture incubator at 37° C. HAEC were subcultured for up to 3 additional passages, using the following protocol:
[0459]Culture medium from confluent T75 flasks of HAEC (6-8 days after seeding) was removed by aspiration, and cells were washed with Dulbecco's phosphate-buffered saline without Mg** or Ca** (commercially available).
[0460]They were then trypsinized as recommended by Biowhittaker.
[0461]The resulting cell suspension was pelleted by centrifugation. The cell concentration was dete...
experiment 1
[0466]Red fluorescent microspheres derivatized with TKPPR, GRGDSP, or BSA (as described above) were diluted at 10 μL / mL EBM medium (Biowhittaker) supplemented with 0.1% (w / v) BSA (Sigma) and 20 μL / mL aprotinin (Sigma). Final bead concentration was 1.95×107 / mL. Unconjugated microspheres were diluted at 5 μL / mL EBM / BSA buffer to give the same microsphere concentration (1.95×107 / mL) achieved with 10 μL / mL of the conjugated preparations. Before starting the assay, bead suspensions were disaggregated in a sonicating bath for 15 min. The wells of an 8-well chamber slide of confluent HAEC were drained of medium and rinsed with 0.5 mL per well of EBM / BSA buffer (without microspheres). To one well each, 250 μL of the following bead solutions (containing 4.9×106 beads) were added: TKPPR-conjugated, BSA-conjugated, and unconjugated. The slide was incubated 30 min on an orbital shaker, drained, then washed once with 0.5 mUwell EBM / BSA buffer, and twice with 0.5 mL / well D-PBS containing 2 mM MgC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com